Multifunctional chiral amine compound, preparation method and application thereof
A chiral amine, multifunctional technology, applied in the direction of sulfonamide preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of limited yield, low non-corresponding selectivity, cinchona The high price of alkaloids and other problems can achieve the effect of high-efficiency catalytic performance and strong synergy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0036]
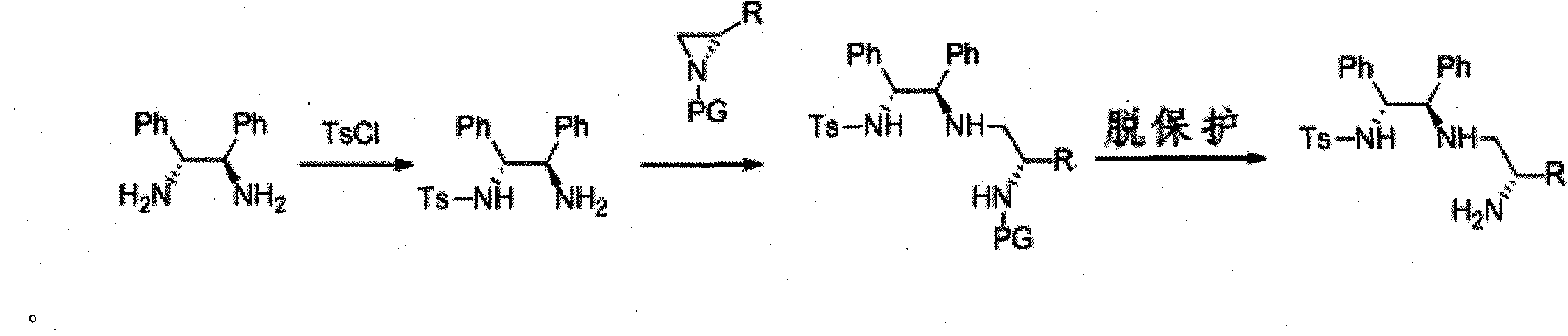
[0037] (R,R)-1,2-Diphenylethylenediamine (4.24g, 20.0mmol) was dissolved in 40mL of anhydrous tetrahydrofuran, and triethylamine (2.7mL) was added in an ice-water bath. Under an ice-water bath, a solution of p-toluenesulfonyl chloride (3.82 g, 20.0 mmol) in 50 mL of anhydrous THF was slowly added dropwise to the above mixture. After the dropwise addition, the mixture was stirred at room temperature for 12 hours, and the solvent was removed from the mixture under reduced pressure. The residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain 7.25 g of white solid with a yield of 90%.
[0038] The above product (2.16, 8.0mmol) was dissolved in 40mL of anhydrous acetonitrile, and 40ml of (S)-N-p-nitrobenzenesulfonyl-1-isopropyl-cycloethyleneimine (2.93, 8.0mmol) was added at room temperature The acetonitrile solution was stirred at 40°C for 12 hours, and the reaction was completed as detected by TLC. The mixture was con...
preparation Embodiment 2
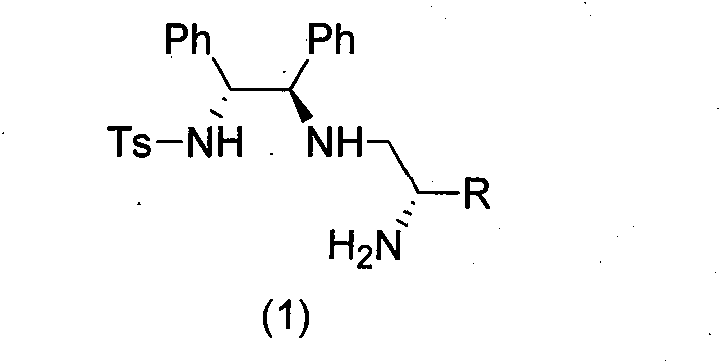
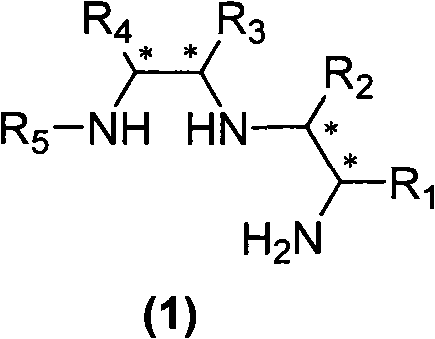
[0044] The difference from Example 1 is that the N-protected ethyleneimine used is (S) N-p-nitrobenzenesulfonyl-1-benzyl-ethyleneimine, and other experimental methods and conditions are the same as in the examples 1, the final product is a white solid (product configuration is R, R, S; R 1 is benzyl, R 2 for hydrogen, R 3 is phenyl, R 4 is phenyl, R 5 is p-toluenesulfonyl), the structure is shown in the figure on the right.
[0045]
[0046] Melting point 164-165℃, optical rotation [α] 20 D -20.3 (c 1.0, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.40-7.38 (m, 2H), 7.32-7.29 (m, 2H), 7.25-7.21 (m, 1H), 7.16-7.11 (m, 5H), 7.07-7.01 (m, 5H) , 6.96-6.93(m, 4H), 4.32(d, J=8.0Hz, 1H), 3.64(d, J=8.0Hz, 1H), 3.03-2.98(m, 1H), 2.72(dd, J=4.4 , 13.2Hz, 1H), 2.50(dd, J=4.0, 11.6Hz, 1H), 2.44-2.39(m, 1H), 2.34(s, 3H), 2.26-2.21(m, 1H). 13 CNMR (100MHz, CDCl 3 ): δ (ppm) 142.5, 139.6, 138.9, 138.6, 137.6, 129.2, 129.1, 128.5, 128.3, 127.8, 127.6, 127.5, 127.4, 127.1, ...
preparation Embodiment 3
[0048] The difference from Example 1 is that the N-protected ethyleneimine used is (S) N-p-nitrobenzenesulfonate
[0049]
[0050] Acyl-1-isobutyl-cycloethyleneimine, other experimental methods and conditions are the same as in Example 1, and the final product is a white solid (the product configuration is R, R, S; R 1 is isobutyl, R 2 is X hydrogen, R 3 is phenyl, R 4 is phenyl, R 5 is p-toluenesulfonyl), the structure is shown in the figure on the right.
[0051] Melting point 120-121℃, optical rotation [α] 20 D -39.3 (c 1.0, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.41-7.39 (m, 2H), 7.16-7.15 (m, 3H), 7.06-7.03 (m, 5H), 6.96-6.94 (m, 4H), 4.33 (d, J=8.0Hz , 1H), 3.64(d, J=8.8Hz, 1H), 2.80-2.74(m, 1H), 2.45-2.41(m, 1H), 2.35(s, 3H), 2.15-2.10(m, 1H), 1.66-1.60(m, 1H), 1.13-1.09(m, 1H), 0.88(d, J=6.8Hz, 3H), 0.86(d, J=6.8Hz, 3H). 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 142.7, 139.6, 138.4, 137.3, 129.1, 128.3, 127.9, 127.6, 127.5, 127.4, 127.2, 127.1, 68.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com