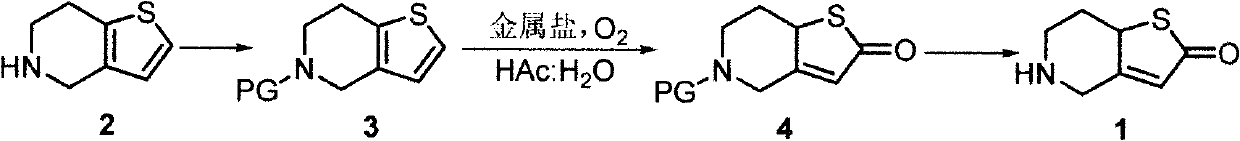Method for preparing prasugrel intermediate and application of method in synthesizing prasugrel
An intermediate and oxidation reaction technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve problems such as industrial production hazards, achieve the effect of increasing the total yield and being suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
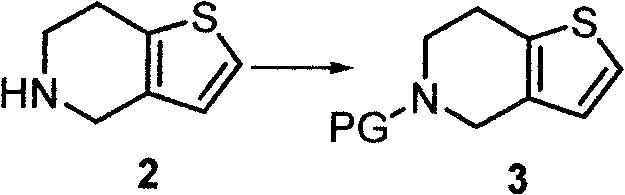
[0025] The preparation method of 5-acetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (compound 3) described in this example is as follows: 4,5,6,7-tetrahydro Thieno[3,2-c]pyridine (7.0 g), diisopropylethylamine (7.2 g), and acetonitrile (30 mL) were mixed, acetic anhydride (5.6 g) was added, and stirred at room temperature for 5 hours. The solution was concentrated to dryness, ethyl acetate and water were added, the liquid was separated, the water phase was extracted twice and the organic phase was combined, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain 5-acetyl - 8.1 g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine, yield 89%.
Embodiment 2
[0027] The preparation method of 5-acetyl-5,6,7,7α-tetrahydrothieno[3,2-c]pyridin-2-one (compound 4) described in this example is: compound 3 (9.1g) , iron trichloride (16.2g), acetic acid (30mL), and water (30mL) were mixed in an autoclave, fed with oxygen, the pressure was at 0.6-0.7MPa, and stirred at room temperature for 8 hours. Adjust the pH of the solution to neutral with dilute sodium hydroxide, add ethyl acetate, separate the layers, extract the water phase twice, combine the organic phases, wash with saturated brine until neutral, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness 7.2 g of 5-acetyl-5,6,7,7α-tetrahydrothieno[3,2-c]pyridin-2-one was obtained with a yield of 73%.
Embodiment 3
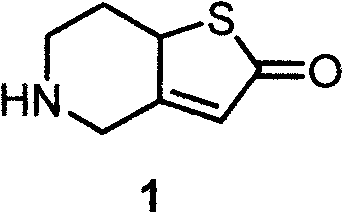
[0029] The preparation method of 5,6,7,7α-tetrahydrothieno[3,2-c]pyridin-2-one (compound 1) described in this example is: compound 4 (9.9g), freshly prepared sodium methoxide Methanol solution (30 mL, containing about 5.4 g of sodium methoxide) was mixed and refluxed for 6 hours. The solution was concentrated to dryness, ethyl acetate and water were added, the layers were separated, the water phase was extracted twice and the organic phases were combined, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain 5, 6, 7,7α-tetrahydrothieno[3,2-c]pyridin-2-one 7.0g, yield 90%; 5,6,7,7α-tetrahydrothieno[3,2-c]pyridine- The 2-ketone was thoroughly ground in ether hydrochloride, crystallized, and filtered to obtain 5,6,7,7α-tetrahydrothieno[3,2-c]pyridin-2-one to obtain 7.1 g of hydrochloride, the yield 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com