Antibody and diagnostic kit
An antibody, truncation mutation technology, applied in the field of diagnosis of hereditary breast and ovarian cancer, to achieve the effect of simple operation, high accuracy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
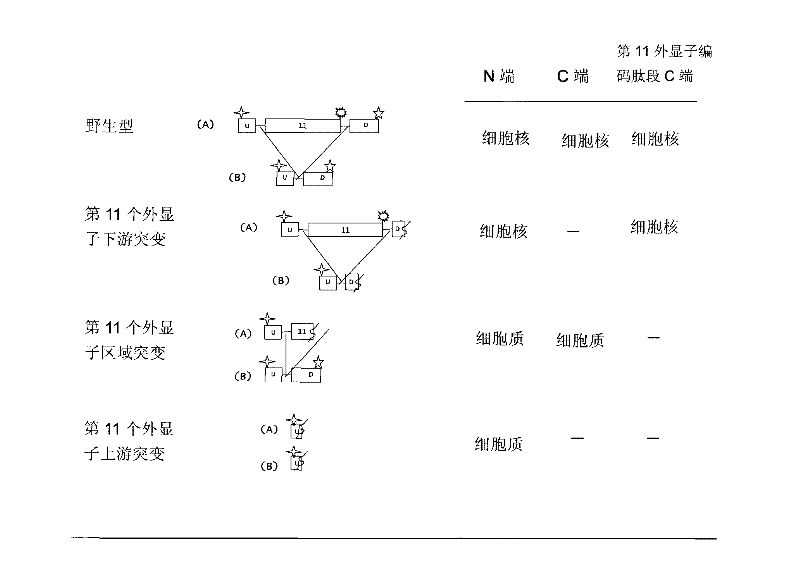
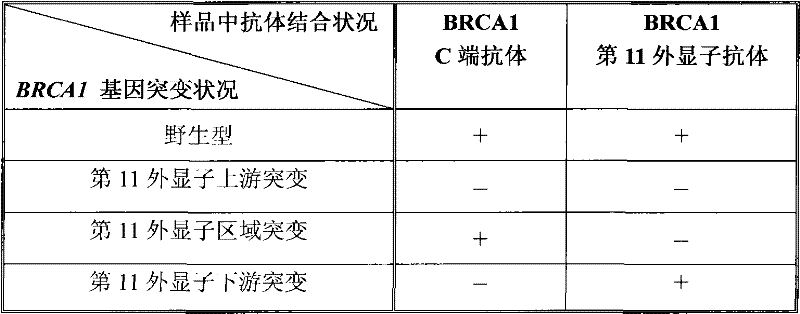
[0055] In order to prepare antibodies that can selectively immunoreact with the C-terminus of the peptide encoded by the 11th exon of the human BRCA1 gene (the 11th exon antibody), we first synthesized the sequence as TGLEENNQEEQSMDSNL (as shown in SEQ ID NO: 3) Shown) the polypeptide, and then link this polypeptide to keyhole limpet halanin (KLH) to immunize mice. Next, the antisera of the immunized mice were subjected to enzyme-linked immunoassay (ELISA) and Western blot detection (Western Blot) of MCF-7 cells, respectively. The mouse serum was screened by immunohistochemical assay (IHC) of MCF-7 cells and tumor sample cells to obtain the optimal antiserum, and the corresponding mice were used for spleen dissection and hybridoma cell preparation. Supernatants from different hybridoma cell lines were screened by Western blot of cell samples, IHC detection of tissue samples from breast cancer patients with mutations in exon 11, and tissue samples from breast cancer patients wi...
Embodiment 2
[0057] Immunofluorescent labeling experiments were carried out on the SKOV-3 cell line without the mutation of the eleventh exon and the cell line UWB1.289 with the mutation of the eleventh exon using the clone monoclonal antibody BZ-98. Specifically, the cells were cultured In a 24-well plate, after washing 3 times with PBS, fix with 4% paraformaldehyde at room temperature for 20 minutes, after washing, rupture the membrane at 37° for 30 minutes. After washing 3 times with PBS, block with 3% BSA for 30 minutes, and then add BZ-98 as primary antibody at 4° overnight. Next, wash 3 times with PBS, add FITC fluorescent secondary antibody diluted with 1% BSA for 30 minutes at room temperature. After washing with PBS for 3 times, stain with Hoechst 33258 for 10 min, and then wash with PBS for 3 times before microscopic examination. The result was that BZ-98 was able to label SKOV-3 cells but not UWB1.289 cells.
Embodiment 3
[0059] MCF-7 and HCC1937 cells were cultured with RPMI-1640 medium containing 10% fetal bovine serum. UWB1.289 cells were cultured with 50% RPMI-1640+50% MEGM medium containing 3% fetal bovine serum. All cells were cultured at 37°C in a humidified environment containing 95% air and 5% carbon dioxide. The cells were broken up, and the cells were collected by centrifugation at 100 g, then, the cells were washed twice with 10 ml of PBS, and fixed with PBS containing 2% formalin for 48 hours. Then wash once with PBS, resuspend in PBS solution containing 2% agarose, embed in paraffin by standard method, and prepare 4 μm paraffin section sections.
[0060] Sections were first deparaffinized in xylene and then dehydrated through graded ethanol prior to immunocytochemical analysis. Each slice was treated with 1% hydrogen peroxide for 20 minutes, then blocked with serum for 20 minutes, and then in a closed container, one of the following primary antibodies was incubated with the slic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com