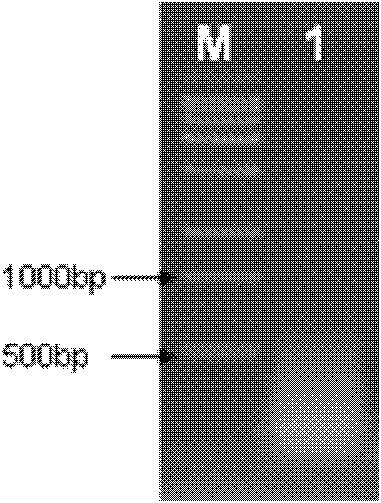PPA-linker-Thanatin fusion protein and preparation method thereof
A technology of fusion protein and protein, applied in the direction of hybrid peptide, recombinant DNA technology, chemical instruments and methods, etc., can solve the problem of low expression activity of fusion protein, and achieve the effect of development and application prospects of major new drug markets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Obtaining the cDNA gene of Pinellia ternata lectin
[0039] The total RNA was extracted from the tubers of Pinellia pedatisecta Schott. According to the instructions for TransScript First-Strand cDNA Synthesis SuperMix, the total RNA of Pinellia pedatisecta Schott was reverse transcribed into single-stranded cDNA.
[0040] The synthesis system of First-Strand cDNA is as follows:
[0041]
[0042] The temperature of the PCR instrument is controlled at 50°C for 30min; the RT Enzyme is inactivated at 85°C for 5min. After cDNA electrophoresis ( figure 1 ) Shows that the cDNA is a diffuse band below 1kb, which is in line with the expected result. Using cDNA as template, according to the complete sequence of Pinellia pedatisecta agglutinin (PPA) mRNA (GeneBank: HM593586.1) reported on NCBI, two pairs of primers PPAL1 and PPAL2 were designed, respectively:
[0043]
[0044] Among them, PPAL1 and PPAL2 were introduced into restriction sites Nco I and Xho I, respectively. R...
Embodiment 2
[0051] Example 2. Cloning of ppa-linker-thanatin recombinant gene
[0052] The ppa encoding Pinellia ternata lectin gene was amplified by the recombinant gene ppa-linker-thanatin through three-step PCR with P1, P2, P3, and P4 primers. The overlapping genes are connected to the 5'end of ppa ORF through P1, P2, and P3, respectively. The PCR products of each step are purified by tapping and cloned onto the T vector. After identification by plasmid PCR and restriction enzyme digestion, they are entrusted to Sangon for sequencing.
[0053]
[0054] The PCR system is as follows:
[0055]
[0056]
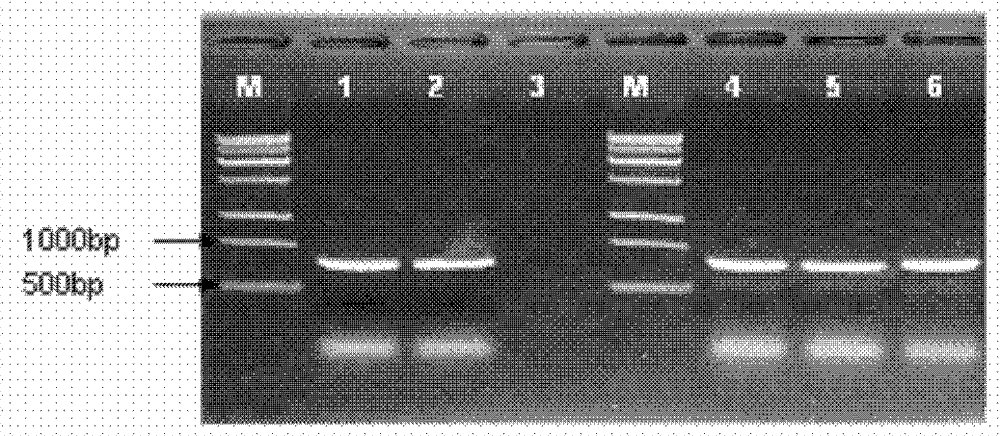
[0057] P1, P4 products (ppal1, primers P1, P4, annealing temperature 55 degrees), P2, P4 products (ppal2, primers P2, P4, annealing temperature 65 degrees), P3, P4 products (ppal3, primers P3, P4, annealing temperature 56 degrees) were respectively connected to pEasy T1 vector, the connection system was the same as in Example 1, and was identified by PCR and restriction digestion ( Image 6 , ...
Embodiment 3
[0060] Example 3. Construction of ppa-linker-thanatin prokaryotic expression vector
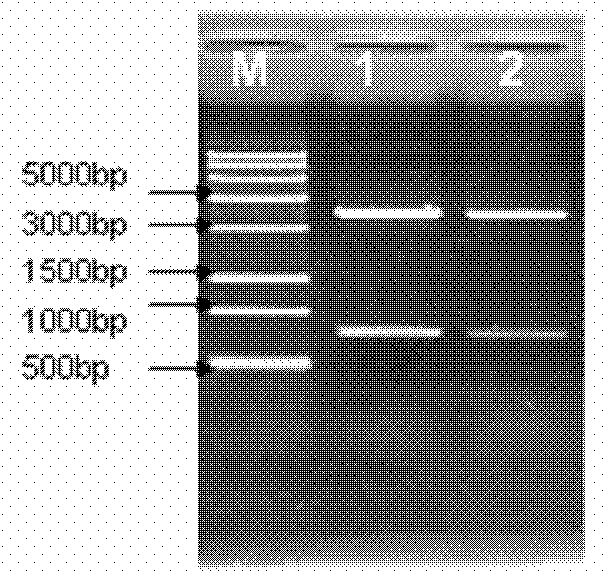
[0061] The ppa-linker-thanatin(pt) gene and pET28a gene were digested with SalI and XhoI to recover the target fragments. Under the action of T4DNA ligase, the expression vector pET28a-pt was constructed, and the vector was transformed into T1 competent cells at 37℃ After culturing for 12 hours, the plasmid was extracted, PCR identification and restriction enzyme digestion identification ( Picture 9 ) Shows that the vector was successfully constructed.
[0062] The amino acid sequence corresponding to the expected recombinant gene is shown in SEQ ID NO: 2 (ie, item 2 of the sequence list).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com