Method for preparing manganese sulfate, manganese nitrate and calcium sulfate
A technology of manganese nitrate and manganese sulfate, applied in the direction of manganese nitrate, manganese sulfate, calcium/strontium/barium sulfate, etc., can solve the problems of impurity enrichment, harsh material requirements, and difficult separation of ferrous sulfate, and achieve simple operation, The effect of less investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
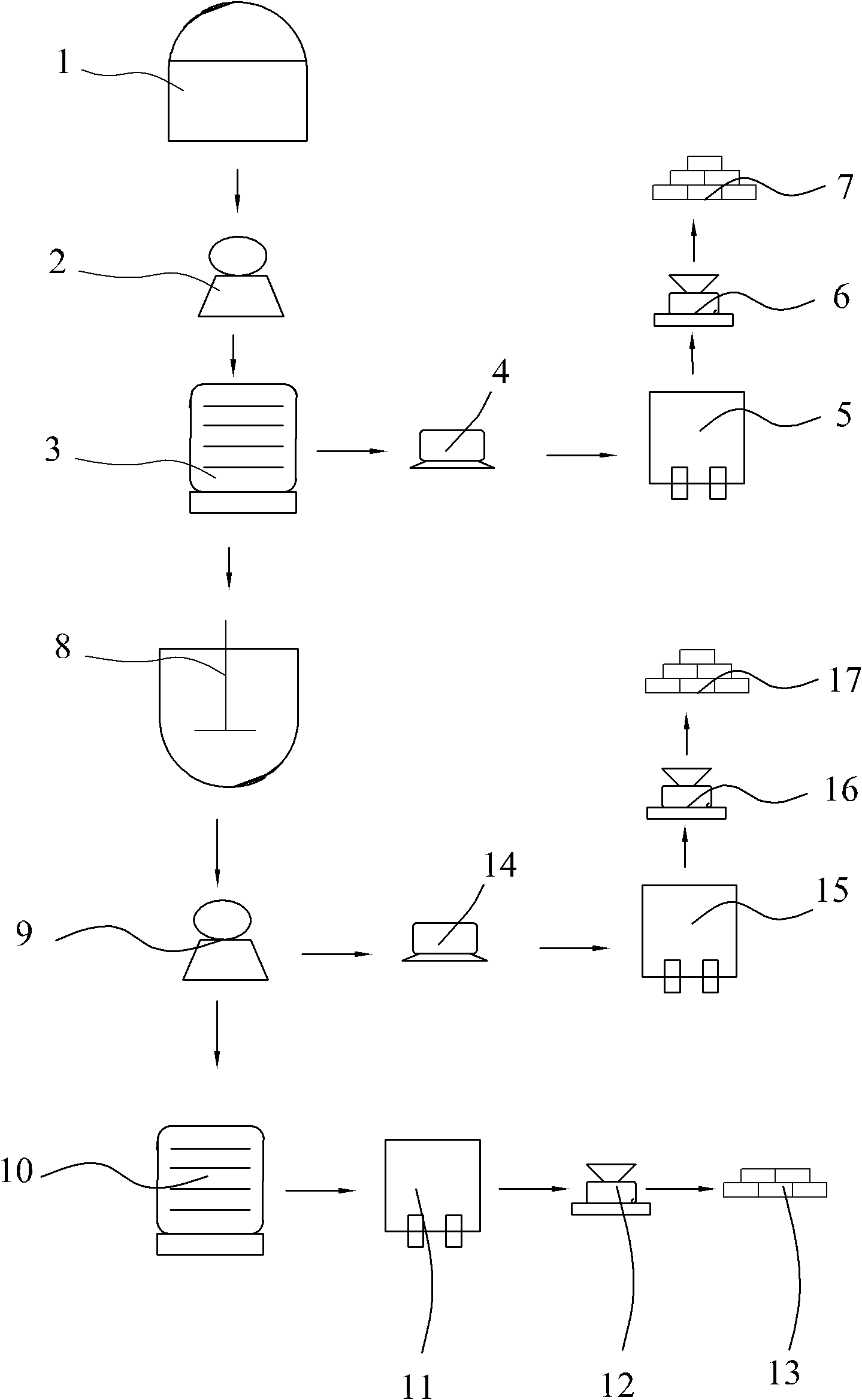
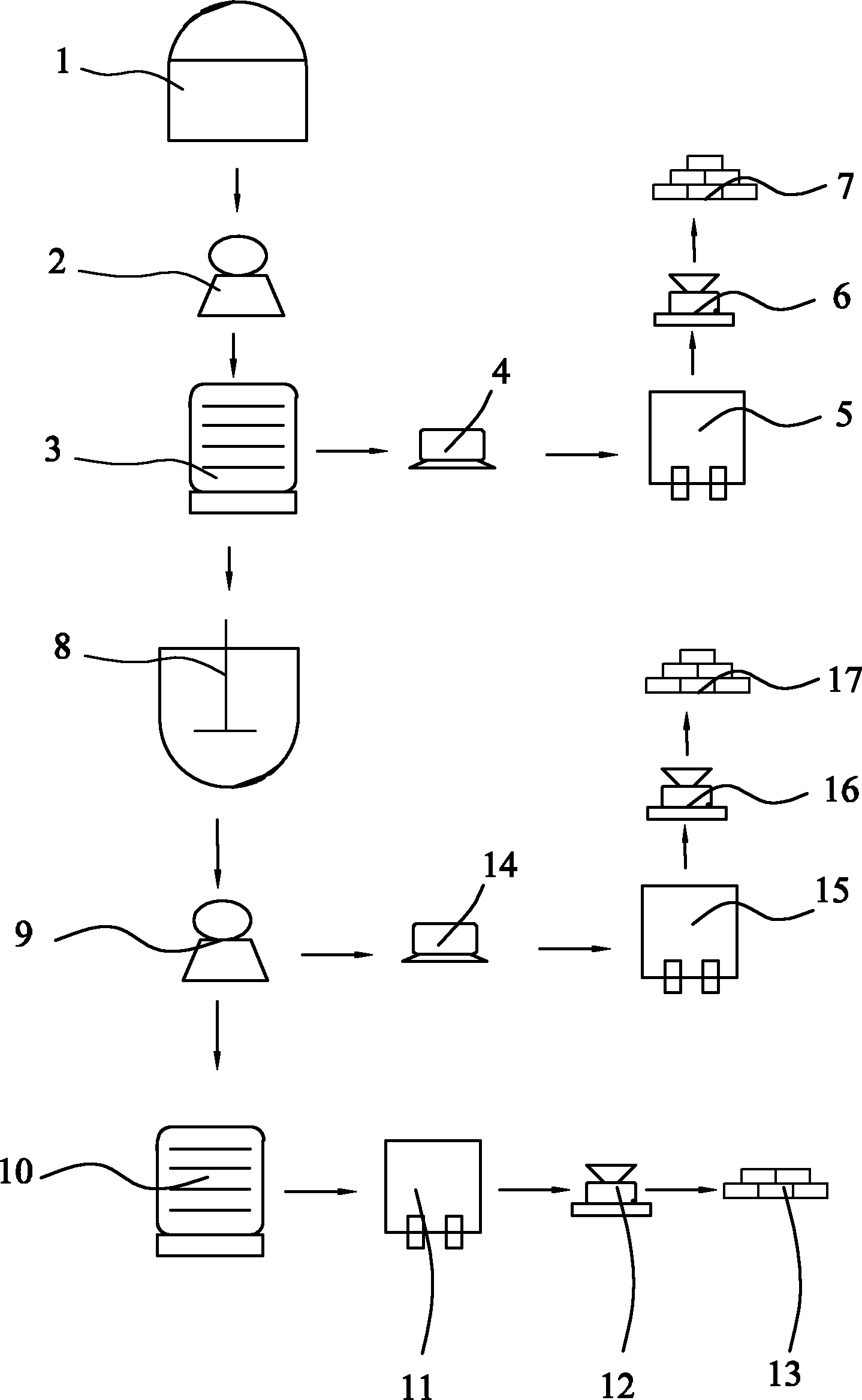
[0023] Reference figure 1 , A method for preparing manganese sulfate, manganese nitrate and calcium sulfate, whose raw materials are waste liquid and manganese ore obtained from the production of titanium dioxide by the sulfuric acid method, including the following specific steps:
[0024] Step A: Take 500kg of waste liquid obtained from the production of titanium dioxide by the sulfuric acid method, adjust the mass concentration of sulfuric acid contained therein to 18%, grind the peridotite into a coarse powder of 100 mesh, and put the waste liquid obtained from the production of titanium dioxide by the sulfuric acid method together with heating The soaking tank (1) of the device is soaked for 8 hours at a soaking temperature of 60°C, and the soaking liquid is filtered through a suction filter (2) to obtain a clear filtrate and filter residue;
[0025] Step B: Add manganese hydroxide to the clarified filtrate to remove impurities and filter to obtain a pure manganese sulfate solu...
Embodiment 2
[0031] Reference figure 1 , A method for preparing manganese sulfate, manganese nitrate and calcium sulfate, whose raw materials are waste liquid and manganese ore obtained from the production of titanium dioxide by the sulfuric acid method, including the following specific steps:
[0032] Step A: Take 500kg of the waste liquid obtained from the production of titanium dioxide by the sulfuric acid method, adjust the mass concentration of sulfuric acid contained therein to 20%, grind the peridotite into a coarse powder of 120 mesh, and put the waste liquid obtained from the production of titanium dioxide by the sulfuric acid method together with heating The soaking tank (1) of the device is soaked for 90 hours at a soaking temperature of 70°C, and the soaking liquid is filtered through a suction filter (2) to obtain a clear filtrate and filter residue;
[0033] Step B: Add manganese hydroxide to the clarified filtrate to remove impurities and filter to obtain a pure manganese sulfate...
Embodiment 3
[0039] Reference figure 1 , A method for preparing manganese sulfate, manganese nitrate and calcium sulfate, whose raw materials are waste liquid and manganese ore obtained from the production of titanium dioxide by the sulfuric acid method, including the following specific steps:
[0040] Step A: Take 500kg of waste liquid from the production of titanium dioxide by the sulfuric acid method, adjust the mass concentration of sulfuric acid contained in it to 30%, grind the peridotite into a coarse powder of 150 mesh, and put it together with the waste liquid from the production of titanium dioxide by the sulfuric acid method with heating The soaking tank (1) of the device is soaked for 10 hours at a soaking temperature of 80°C, and the soaking liquid is filtered through a suction filter (2) to obtain a clear filtrate and filter residue;
[0041] Step B: Add manganese hydroxide to the clarified filtrate to remove impurities and filter to obtain a pure manganese sulfate solution;
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com