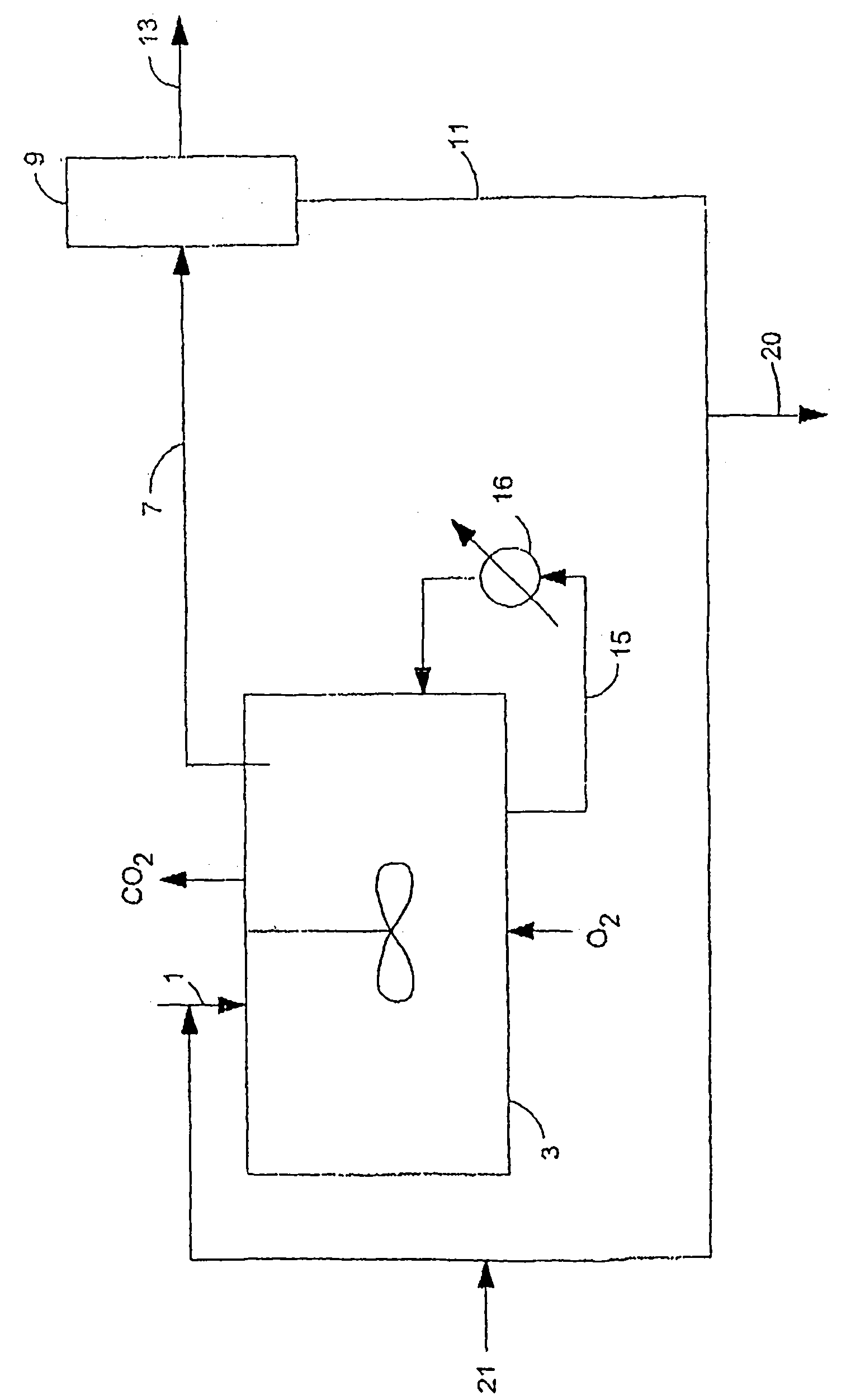
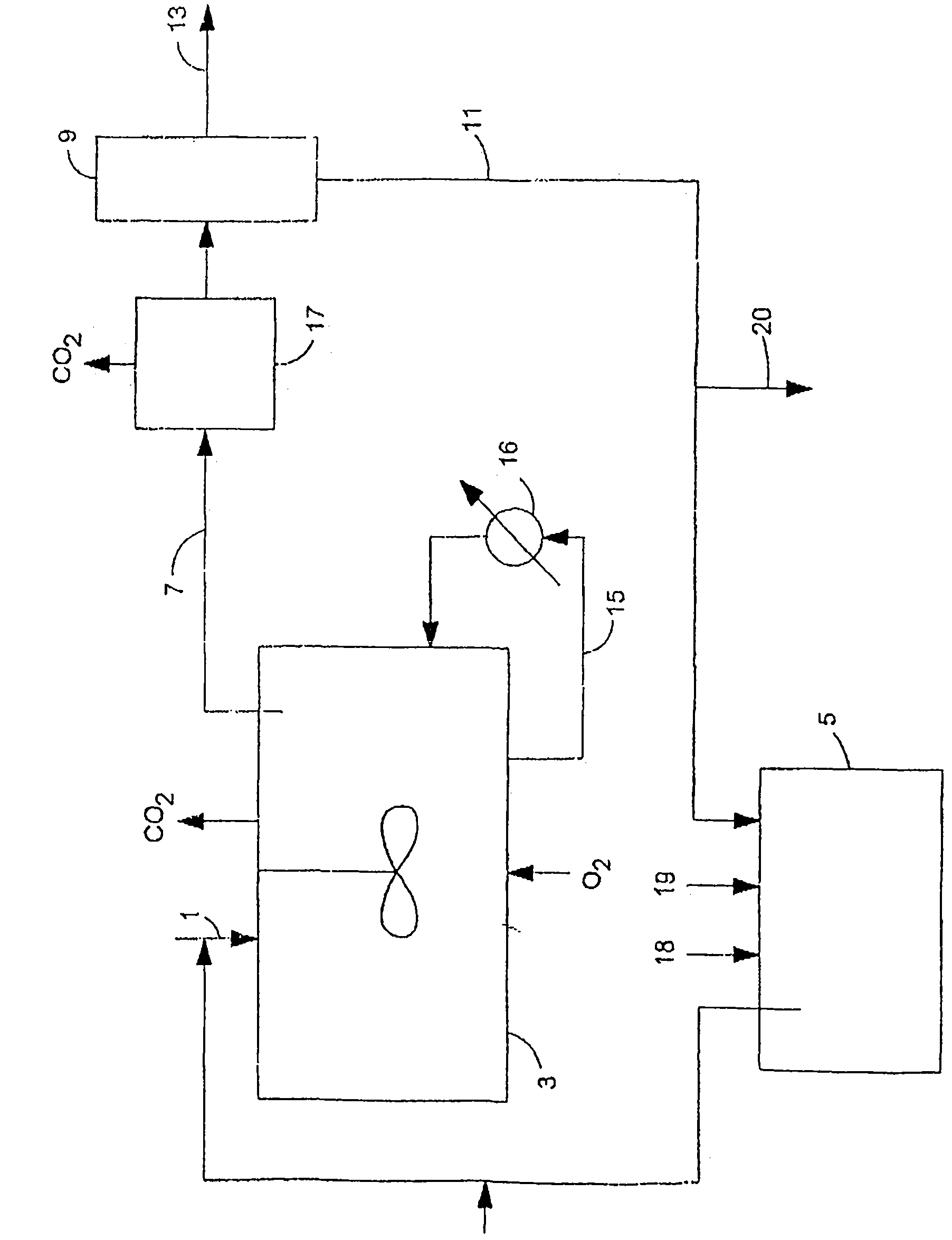
Reaction systems for making n-(phosphonomethyl)glycine compounds
A kind of technology of phosphonomethyl and glycine, applied in the field of liquid phase oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0861] Example 1 Measuring the Pore Volume of Carbon Supports
[0862] Data were acquired using a Micromeritics ASAP 2000 surface area and pore volume distribution meter. Total surface area determination involves exposing a known weight of a solid to a pressure of a non-specifically adsorbed gas at a constant temperature, eg, the temperature of liquid nitrogen, ie -196°C. During equilibrium, gas molecules leave the host gas and adsorb onto surfaces, causing the average number of molecules in the host gas to decrease, which in turn lowers the pressure. Record the saturation vapor pressure p of the gas with oThe relative pressure p at equilibrium varies with the change. By combining this pressure drop with the volume of the container and sample, the amount of adsorbed gas (ie, the number of molecules) can be calculated by applying the ideal gas law. These data are at relative pressures of about 0.1 to 0.3 (p / p o ), in which case the Brunauer, Emmett and Teller (BET) equation...
Embodiment 2
[0863] Example 2 High temperature deoxidation of carbon support
[0864] Deoxygenated carbon supports can be obtained with any carbon support using the high temperature deoxygenation procedure described in the examples below.
[0865] Use NH 3 / H 2 One-step high temperature deoxygenation of O gas #1
[0866] Activated carbon support (2.5 g) was packed into a 1.9 cm (inner diameter) x 40.6 cm long quartz tube. The tube is passed through the 70-100ml / min N 2 Gas stream sprayed through 10% NH at 70°C 4 The gas flow obtained by the aqueous OH solution is communicated. The quartz tube was then placed in a preheated 30.5 cm tube furnace, pyrolyzed at 930 °C for 60 min, and then dried in N 2 Cool to room temperature under atmosphere.
[0867] Use NH 3 / H 2 Single-step high-temperature deoxygenation of O gas #2
[0868] Activated carbon support (3.55 g) was put into a 1.9 cm (inner diameter) x 35.6 cm long quartz tube. The tube was mixed with 50ml / min NH ...
Embodiment 3
[0875] Embodiment 3, platinum is deposited on the surface of carbon carrier
[0876] 20 g of NUCHAR activated carbon SA-30 (Westvaco Corp. Carbon Department, Covington, VA) was slurried in 2 L of water for 2 hours. Then, 2.81 g of H dissolved in about 900 ml of water was added dropwise over 3-4 hours. 2 PtCl 6 . After adding H 2 PtCl 6 After solution, the slurry was stirred for an additional 90 minutes. The pH of the slurry was then readjusted to 10.5 using NaOH and stirred for an additional 10-14 hours. The resulting slurry was filtered and washed with water until the filtrate reached a constant conductivity. The wet cake was dried at 125°C under vacuum for 10-24 hours. After reduction of this material, 5% Pt / C was obtained.
[0877] It should be appreciated that the above procedure can also be used to deposit platinum onto the surface of other carbon supports.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com