Catalyst for preparing cyclohexanol by hydration of cyclohexene and its preparation and application method
A cyclohexene hydration and catalyst technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve complex separation of catalysts and reaction products, and difficult corrosion problems Solve the problems of homogeneous catalyst corrosion and other problems, and achieve the effect of easy regeneration and repeated use, no corrosion of equipment, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
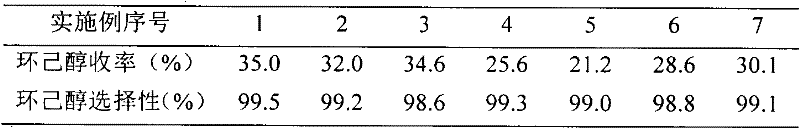
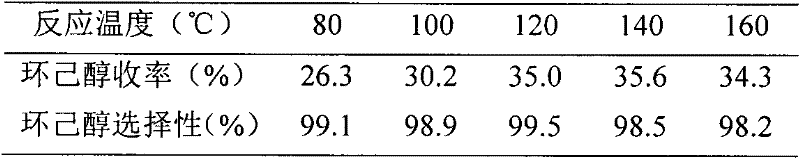
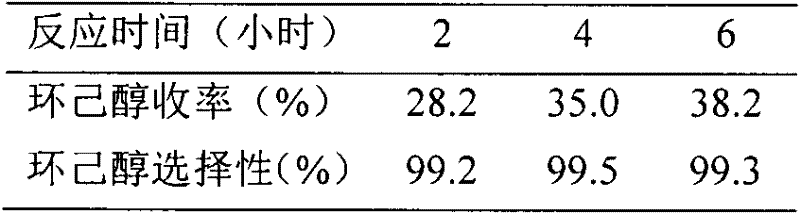
Examples
Embodiment 1
[0018] (1) Place a trimethylamine aqueous solution containing 1.0 mole of trimethylamine and 1.0 mole of 1,4-butane sultone in a flask, add 50 g of HZSM-5 to it, and react at 60°C for 48 hours. After the reaction mixture was taken out, the water was distilled off under reduced pressure to obtain a white solid. The solid was washed with absolute ethanol, toluene and anhydrous ether in sequence, and then vacuum dried at 80°C to a constant weight.
[0019] (2) Put the solid obtained in the first step into a flask, add 1.0 mol concentrated sulfuric acid at 5°C, raise the temperature to 60°C, and react for 5 hours. Wash with deionized water, filter, and vacuum-dry to constant weight at 80°C to obtain HZSM-5 supported B acid ionic liquid N, N, N-trimethyl-N-sulfobutyl-ammonium hydrogen sulfate catalyst.
Embodiment 2、3
[0021] The preparation method is the same as in Example 1, except that the amount of 1,4-butane sultone in step (1) is changed to 0.8 mol and 1.2 mol, respectively, to obtain the required HZSM-5 load B acidity of the present invention. Ionic liquid N, N, N-trimethyl-N-sulfobutyl-ammonium hydrogen sulfate catalyst.
Embodiment 4、5
[0023] The preparation method is the same as in Example 1, except that the amount of HZSM-5 in step (1) is changed to 100 g and 150 g respectively to prepare the HZSM-5 supported B acidic ionic liquid N, N, N-Trimethyl-N-sulfobutyl-ammonium hydrogen sulfate catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com