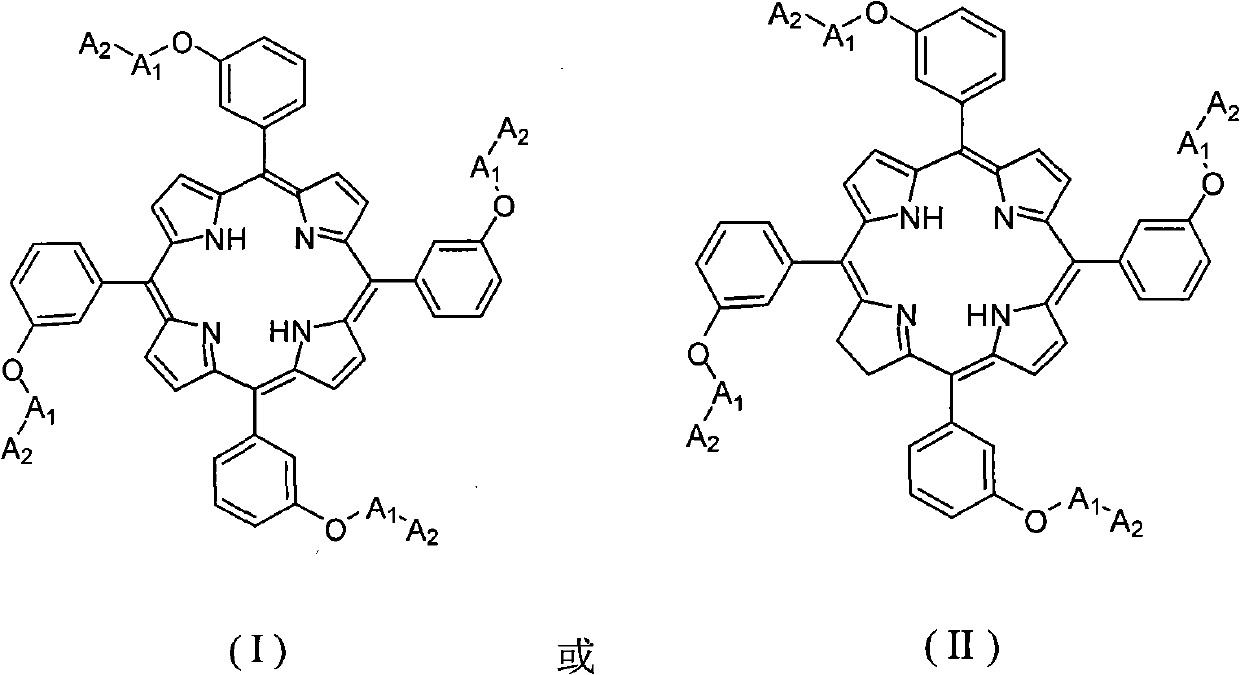
A kind of dendritic compound containing porphyrin or chlorin and application thereof
A technology of chlorin and compounds, applied in the field of dendrimers, which can solve problems such as low solubility, limited application range of compounds, and insufficient selectivity of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Its preparation method is as follows:
[0081]
[0082] 1. Preparation of compound (4):
[0083] Take compound (3) (28g, 19mmol), put it in a 1000ml round bottom flask, add 400mL of absolute ethanol, and then add Raney Ni prepared from 30g of nickel-aluminum alloy. Hydrogenation reaction at normal temperature and pressure. After the reaction was complete, Raney Ni was removed by filtration, and the solvent was evaporated by rotary evaporation to obtain 27 g of white solid. Yield 100%. m.p.: 180-181°C. LC-MS: M+1, 1440.3.
[0084] 2. Preparation of compound (5):
[0085] Get compound (4) (25g, 17mmol) and place in the round bottom flask, add 150mL toluene, Et 3 N (20 mL, 138 mmol) After compound (4) was completely dissolved, succinic anhydride (13.8 g, 138 mmol) was added. Heated to reflux for 12 hours. After the reaction, the solvent was evaporated by rotary evaporation, 200 mL of ethyl acetate was added, washed with 50 mL*4 saturated brine, and dried over anh...
Embodiment 2
[0091] Its preparation method is as follows:
[0092]
[0093]
[0094] 1. The preparation of compound (8):
[0095] Weigh compound (7) (30g, 136mmol), NaBH 4 (30g, 830mmol), placed in a three-necked flask, added 1000mL dry THF, stirred at room temperature for 30 minutes, added dropwise 240ml (CH 3 ) 3 SiCl (1.6mol), after the dropwise reaction was heated under reflux for 18 hours. The reaction solution was cooled, and water was added dropwise in an ice-water bath to quench the reaction. The solvent was evaporated, methanol was added to dissolve, and the filtrate was concentrated to obtain a viscous liquid, which was directly used for the next reaction.
[0096] 2. Preparation of compound (9):
[0097] Place the crude product of compound (8) obtained in the previous step reaction in a round bottom flask, add 300mL of methanol to dissolve, then add 60mL of triethylamine, dropwise add (Boc) 2 O (112 g, 0.45 mol). Stir at room temperature for 4 hours after dropping, ...
Embodiment 3
[0111] Its preparation method is as follows:
[0112]
[0113] 1. Preparation of compound (16):
[0114] Weigh compound (5) (3g, 1.95mmol), place in a round bottom flask and add 30mL THF, add compound (2) (83mg, 0.12mmol) after compound (5) dissolves completely, dicyclohexylcarbodiimide (DCC) (4.6g, 23mmol), 4-(N,N-dimethylamino)pyridine (DMAP) (0.8g, 5.85mmol) and p-toluenesulfonic acid (TsOH) (1.04g, 5.5mmol) were stirred at room temperature After 12 hours, the solid was removed by suction filtration, the filtrate was concentrated, and 30 mL of CH 2 CL 2 Then add 4.6g of DCC, 0.8g of DMAP, 1.04g of TsOH, stir the reaction at room temperature, stop the reaction after seven days, filter, and the filtrate is first washed with saturated NaHCO 3 solution (10mL*3), then washed with saturated NaCl solution (10mL*3), and then washed with anhydrous NaCl 2 SO 4 Drying and column chromatography (gradient elution: THF:PE=1:4, THF:PE=1:3, THF:PE=1:2) yielded 220 mg of the target ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com