An active targeting liposome delivery system for tumors in situ and lymphatic metastases
A carrier system and lymphatic transfer technology, which can be used in liposome delivery, anti-tumor drugs, medical preparations with inactive ingredients, etc., and can solve problems such as affecting the effect of targeted therapy, tissue damage, and necrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
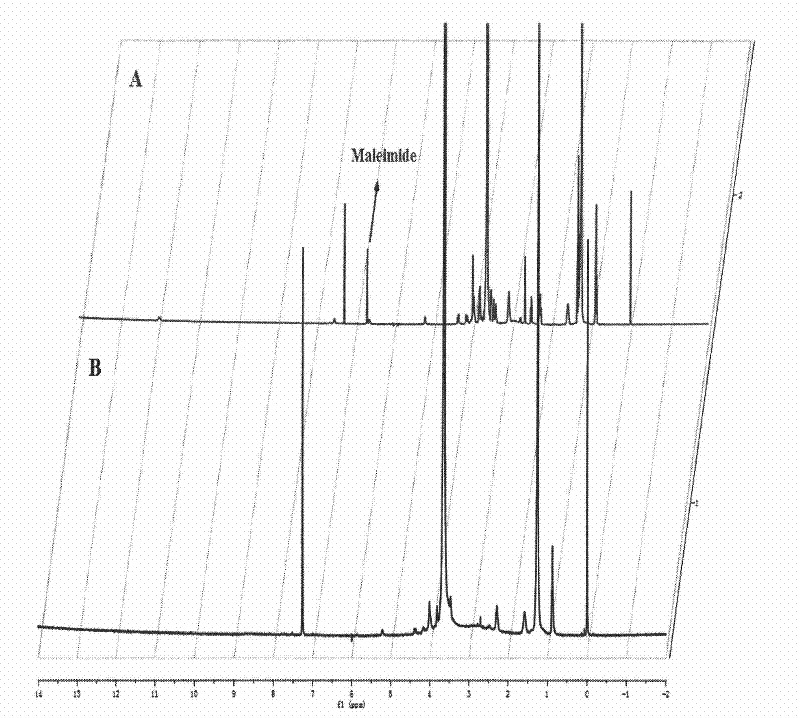
[0044] Synthesis, purification and characterization of LyP-1-FAM and LyP-1-PEG-DSPE
[0045] 1. Synthesis, purification and characterization of LyP-1-FAM
[0046] Weigh 0.4167g of Boc-Cys(Mbzl)-PAM resin (degree of substitution: 0.6mmol / g) in a peptide bottle, swell the resin with DMF (N,N-dimethylformamide), and drain it after 20 minutes . Add TFA (trifluoroacetic acid) about twice the volume of the resin to stir the reaction, remove the TFA, then add TFA and operate in the same way once to remove the Boc protecting group. Activation of Boc-Gly with HBTU (benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate) in DMF and DIEA (N, N-diisopropylethylamine), After the resin was washed with DMF, Boc-Gly activation solution was added, and the reaction was shaken. After the reaction was completed, the reaction solution was removed, and the resin was washed with DMF. Subsequently, the remaining amino acids were sequentially connected according to the LyP-1 sequence by...
Embodiment 2
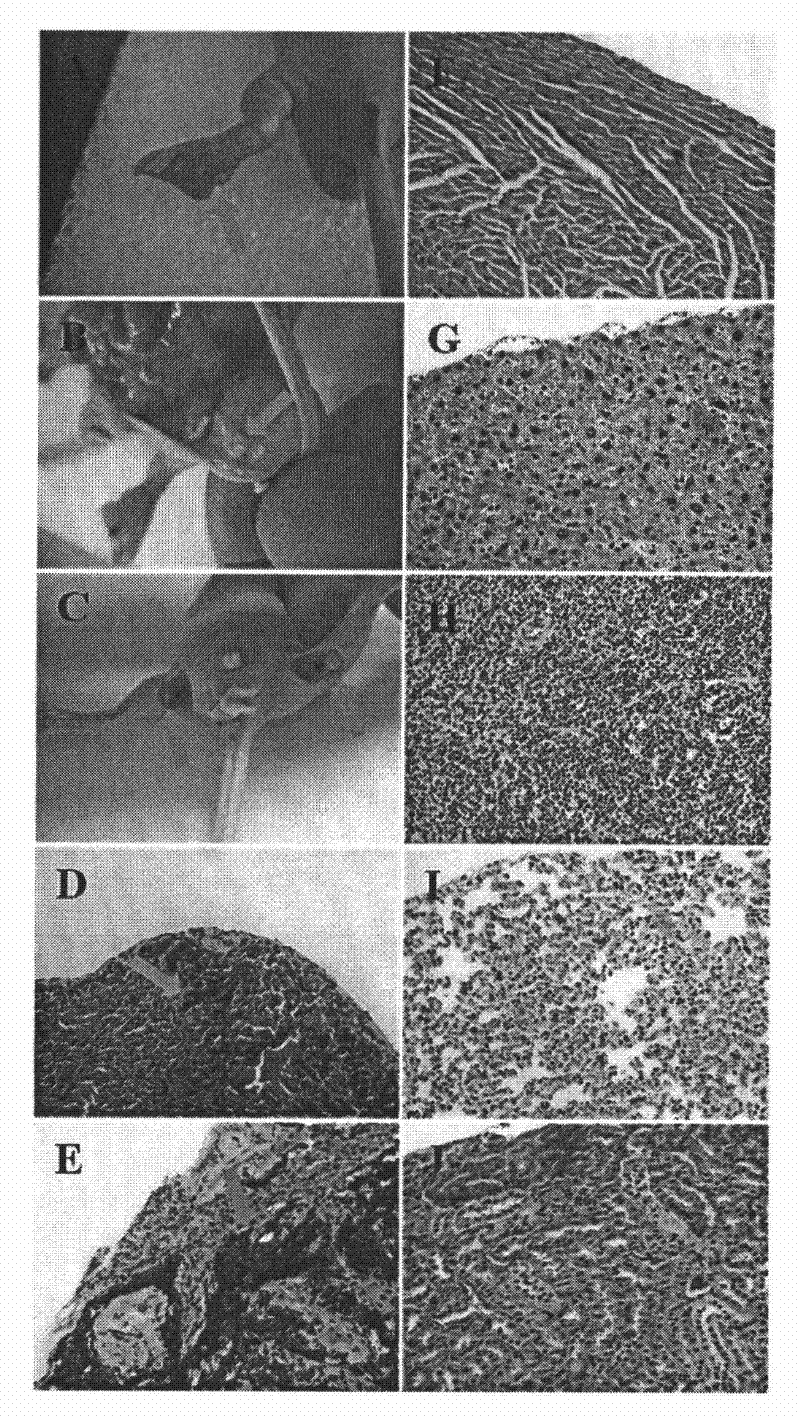
[0055] Construction of animal models of orthotopic tumors and lymphatic metastatic tumors
[0056] Nude mice were subcutaneously inoculated with MDA-MB-435 tumor cells in both hind footpads or single hind footpad, at a concentration of 1×106 cells / footpad. They were raised at the SPF level, and the lymph nodes and major organs at all levels were taken for pathological examination 3 and 6 weeks after inoculation to determine the time of lymphatic metastasis of the tumor and provide reference for tumor staging.
Embodiment 3
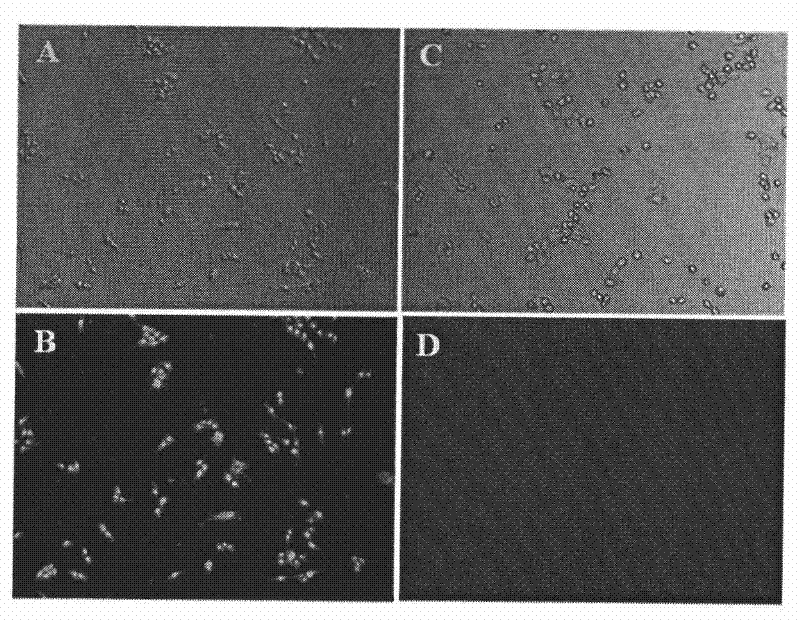
[0058] In vivo and in vitro targeting verification of LyP-1
[0059] 1. In vitro tumor cell targeting verification of LyP-1
[0060] Take the monolayer cultured MDA-MB-435 cells in the logarithmic growth phase, digest the monolayer cultured cells with 0.25% trypsin and 0.025% disodium edetate, and prepare with DMEM culture medium containing 10% fetal bovine serum Single cell suspension, 1×10 per well 5 Cells were inoculated in a 24-well culture plate with a volume of 1 ml per well, and the culture plate was moved into a carbon dioxide incubator, and cultured overnight at 37°C, 5% carbon dioxide and saturated humidity conditions, so that the cells adhered to the wall. On the next day, a series of FAM and LyP-1-FAM solutions with different concentrations were prepared with DMEM medium containing 1% fetal bovine serum. Aspirate the culture medium in the culture plate, add a series of solutions of FAM and LyP-1-FAM, incubate at 37°C for 4 hours, and discard the supernatant. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com