A kind of synthetic method of everolimus
A synthesis method and intermediate technology, which are applied in the field of medicinal chemistry synthesis, can solve the problems of low product yield and the like, and achieve the effect of improving the overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
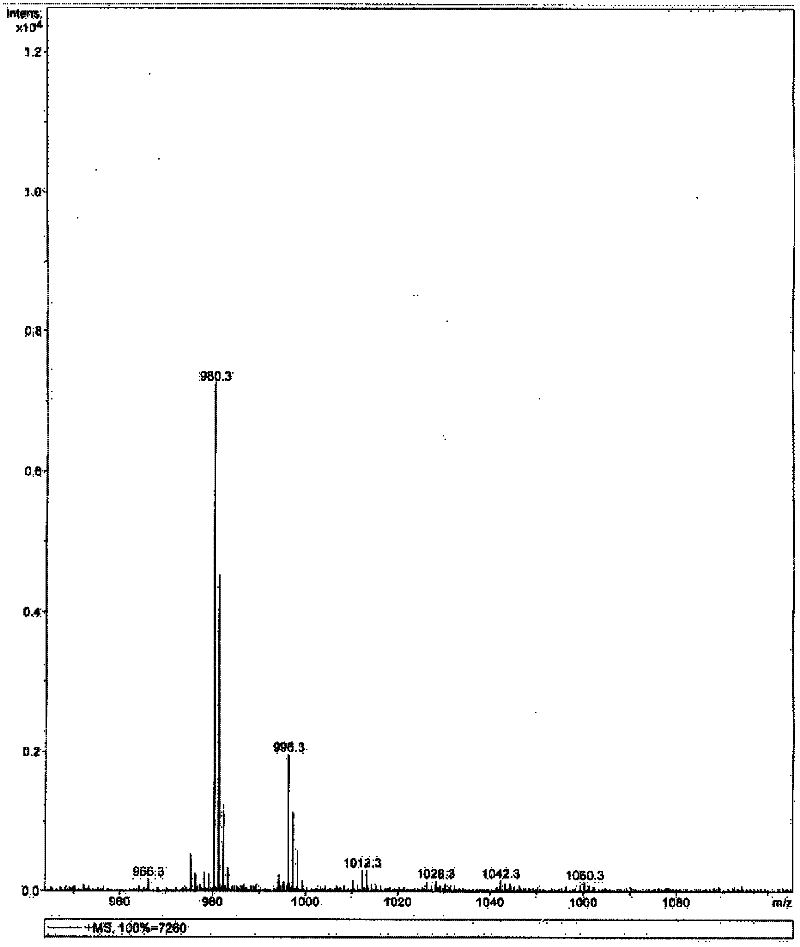
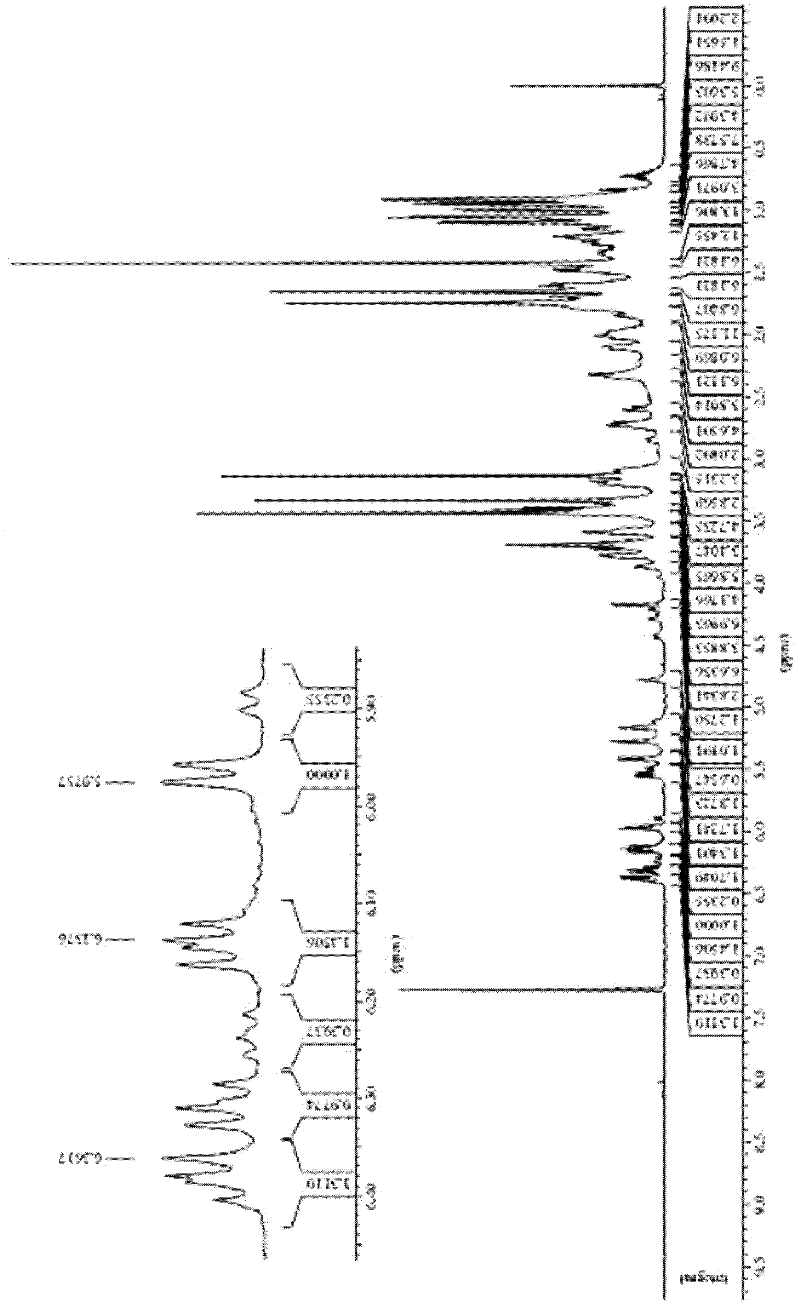
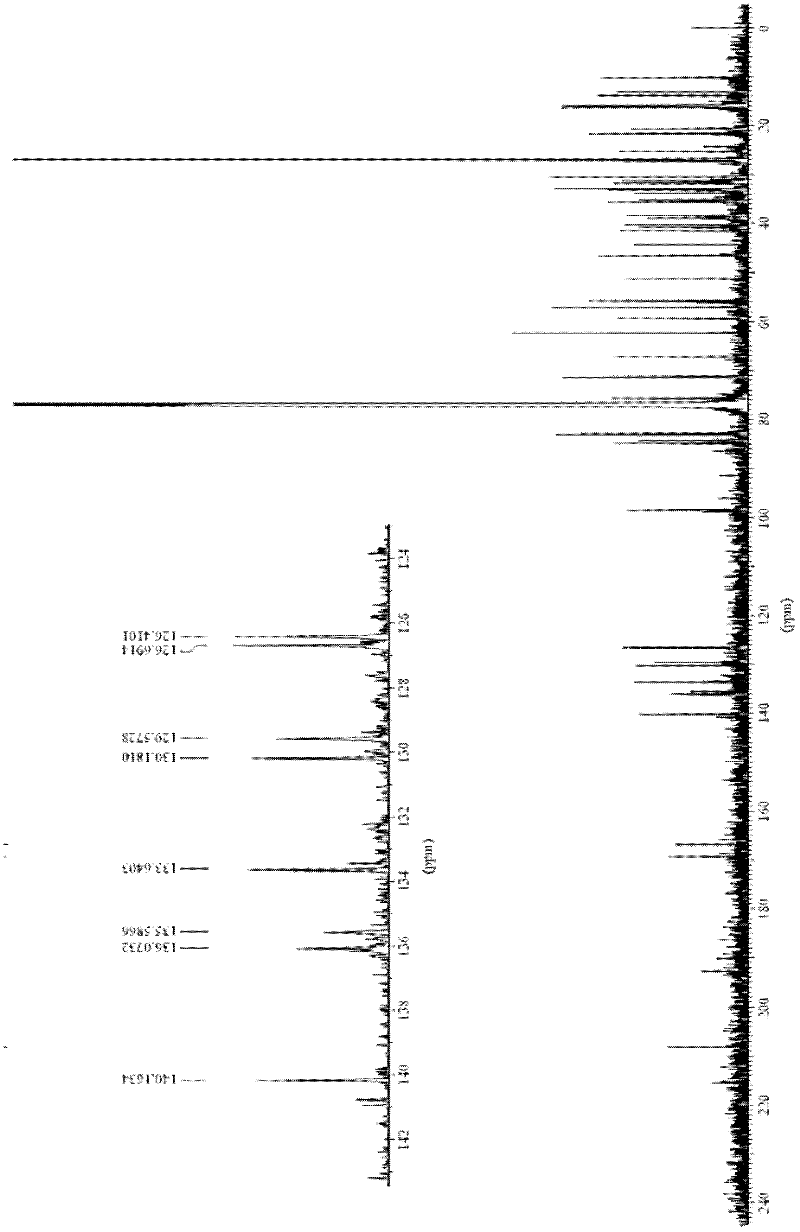
Image
Examples
Embodiment 1
[0052] The synthetic method of the everolimus of the present embodiment comprises the following steps:
[0053] (1) Preparation of everolimus intermediate 02:
[0054] In a 300mL multi-necked flask, weigh 10g of rapamycin under the condition of dryness and nitrogen protection, add it into 80mL of dichloromethane organic solvent, stir to dissolve, and then add 2,6-lutidine 10g, lower the temperature to -20°C, add 14g of trifluoromethanesulfonic anhydride dropwise and stir, after the dropwise addition, keep the reaction for 3h. HPLC detects reaction, when raw material reaction completes. 100 mL of saturated brine was added dropwise, the layers were extracted, and the organic layer was washed several times with 100 mL of saturated brine until nearly neutral, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 10 g of everolimus intermediate 02. The yield of intermediate 02 was 87.4%.
[0055] (2) Preparation of everolimus intermediate 03:
...
Embodiment 2
[0061] The 31-hydroxyl of the raw material rapamycin in this embodiment is protected with dimethyl tert-butyl silyl ether, that is, the raw material is a kind of rapamycin derivative (31-TBDMS-pamycin). The synthetic method of everolimus comprises the following steps:
[0062] (1) Preparation of everolimus intermediate 02
[0063] In a 300mL multi-necked flask, under the condition of dry anhydrous and nitrogen protection, dissolve 11.25g of 31-TBDMS-pamycin in 80mL of dichloromethane, add 10g of 2,6-lutidine, and cool down to -20℃ , Add 14 g of trifluoromethanesulfonic anhydride dropwise, after the addition, keep the temperature for 3 h. HPLC detects that the reaction is complete. 100 mL of saturated brine was added dropwise, and the layers were extracted. The organic layer was washed with 100 mL of saturated brine several times until nearly neutral, and dried over anhydrous sodium sulfate. Concentration under reduced pressure yielded 12.1 g of everolimus intermediate 02. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com