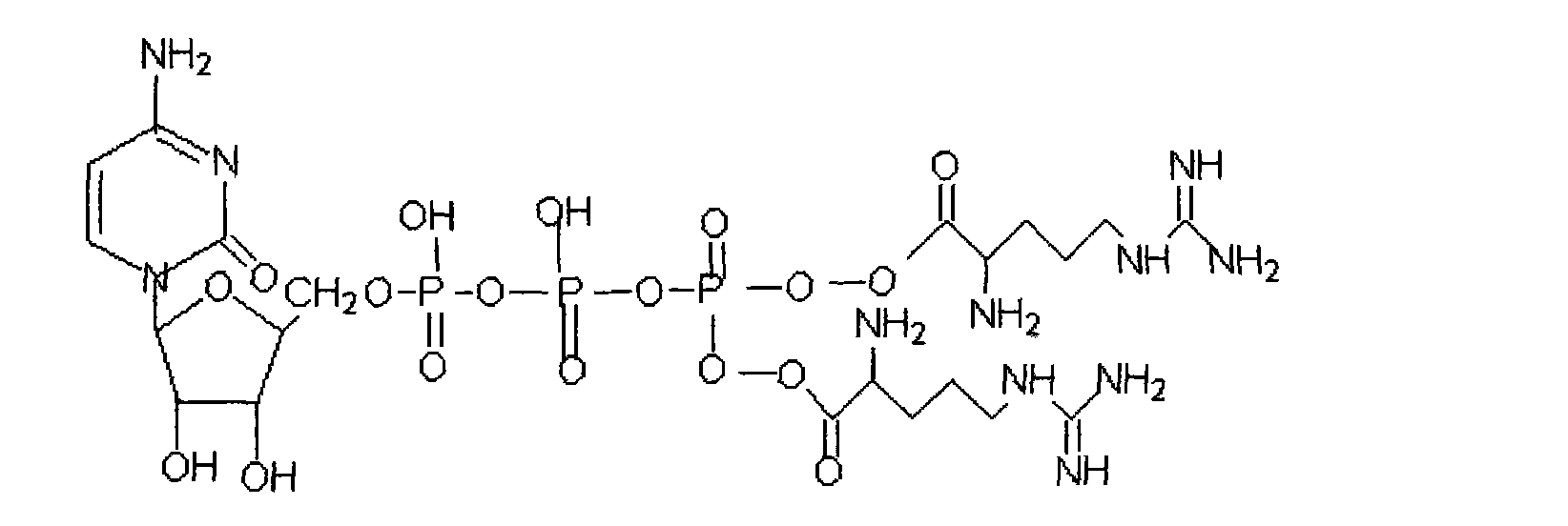
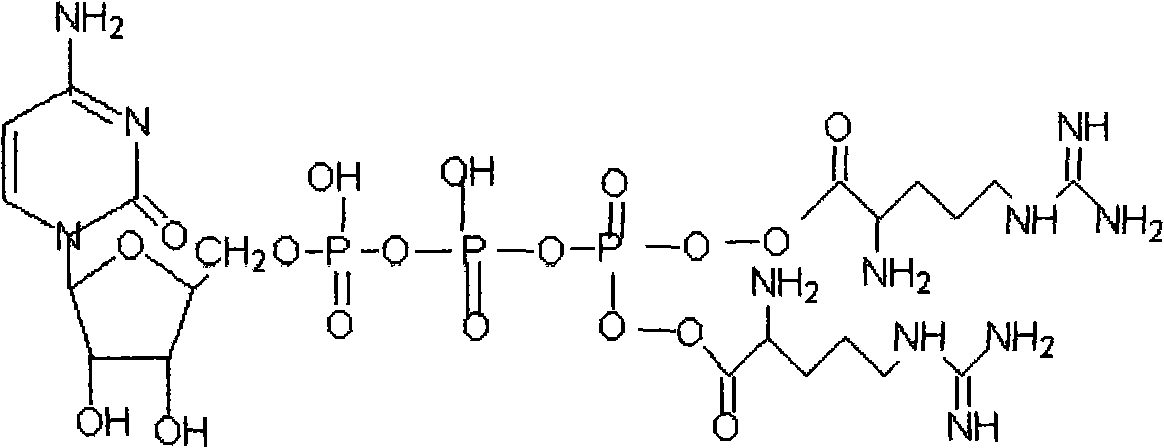
A kind of β-cytidine nucleoside-5'-arginine triphosphate derivative ester and its preparation method and application
A technology of arginine triphosphate and cytidine nucleoside, which is applied to the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., and can solve problems such as mental disorders, family and social burdens, and difficulties in treatment and care
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The ultraviolet analysis of embodiment 1-beta-cytidine-5'-arginine triphosphate
[0043] Adopt ultraviolet-visible spectrophotometry to analyze and measure the above-mentioned compounds, take compound 1 to prepare the test solution, and there is maximum absorption at the wavelength of 280 ± 2nm;
[0044] Table 1: Maximum absorption wavelength position and absorbance ratio range:
[0045]
[0046] Take compound 2 to prepare the test solution, which has maximum absorption at the wavelength of 250 ± 2nm;
[0047] Table 2: Maximum absorption wavelength position and absorbance ratio range:
[0048]
[0049]
Embodiment 2
[0050] The HPLC analysis of embodiment 2-beta-cytidine-5'-arginine triphosphate:
[0051] The detection wavelength is 280nm, the mobile phase is 0.02mol / L phosphate buffer: methanol (75:25), the number of theoretical plates is not less than 2000, the resolution is greater than 1.5, the flow rate is 1.0ml / mim, and the running time is 20 minutes , the injection volume was 10 μl / time, and each operation was equilibrated at intervals of 10 minutes.
[0052] Table 1: Retention times of Compound 1
[0053]
[0054]The detection wavelength of the ultraviolet detector is 272nm, the chromatographic column adopts C18, 3.9×150mmID, the particle size of 4μm, with 0.02mol / L phosphate buffer: methanol (75:25) as the mobile phase, the number of theoretical plates is not less than 2000, the resolution is greater than 1.5, the flow rate is 1.00ml / mim, the running time is 35 minutes, the injection volume is 10μl / time, the column temperature is controlled at 25°C, and the interval between ea...
Embodiment 3
[0057] Example 3-Pharmacology of β-cytidine-5'-arginine triphosphate
[0058] β-cytidine nucleoside-5'-arginine triphosphate can promote the synthesis and metabolism of phospholipids, fats, nucleic acids and proteins in cells, regulate the synthesis and reconstruction of cell biofilm structures, enhance the activity and regeneration of vascular cells and Repair ability, improve cell resistance to damage, promote the substance and energy metabolism of nerve cells, support the survival of neurons, delay the aging of nerve cells and hardening of blood vessels, and can pass through the blood-brain barrier and directly enter the nerve cells, affecting the respiratory chain, Improve brain cell ischemia and hypoxia.
[0059] It is used to treat nervous system diseases and vascular sclerosis caused by various reasons. Cytidine-5”-cytidine triphosphate, which participates in the synthesis and construction of the body’s membrane structure, can be catabolized into cytidine monophosphate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com