The synthetic method of dichloroacetate
A technology of dichloroacetate and synthesis method, which is applied in the field of compound synthesis, can solve the problems that cannot be used as raw material medicine, product quality is uncontrollable, and the synthesis process does not conform to the production process of raw material medicine, so as to achieve controllable quality and easy operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Sodium Dichloroacetate
[0027] Step 1.1 Esterification of 2,2'-dichloroacetyl chloride with 9-fluorenylmethanol to obtain fluorenylmethyl dichloroacetate
[0028] Add 5.00 g of 9-fluorenemethanol and 4.51 g of 2,2'-dichloroacetyl chloride to 20 ml of toluene solution, mix and heat to 110°C for 2 hours under reflux. After the reaction is complete as detected by TLC, the reaction solution is placed in After vacuum concentration at 70°C, 10 ml of toluene was added for concentration, and finally a solid crude product was obtained.
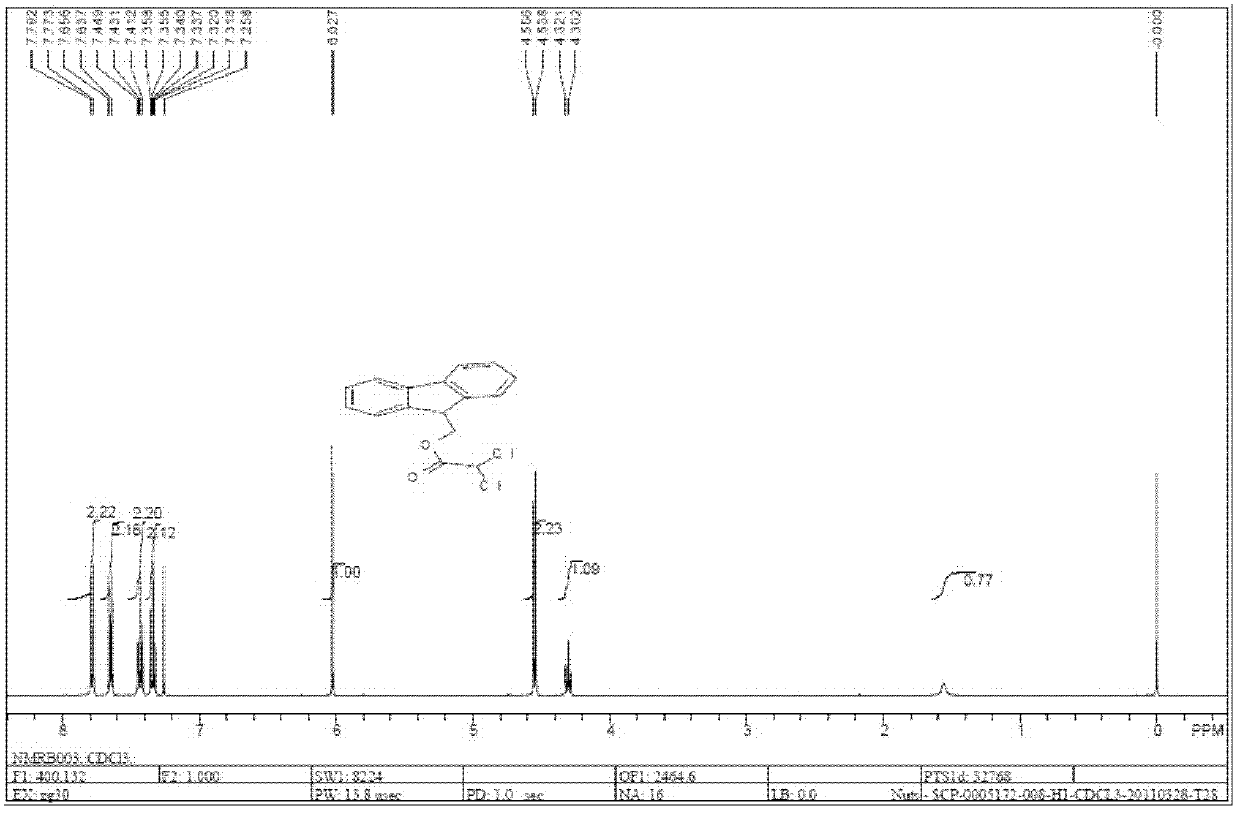
[0029] The obtained above crude product was crystallized at 55°C in 50 ml of acetone and petroleum ether mixed solvent with a volume ratio of 1:9 to obtain 6.01 g of the formula The compound, fluorenylmethyl dichloroacetate. After concentrating and recrystallizing the mother liquor, 0.69g of formula The compound, the yield is 86%.
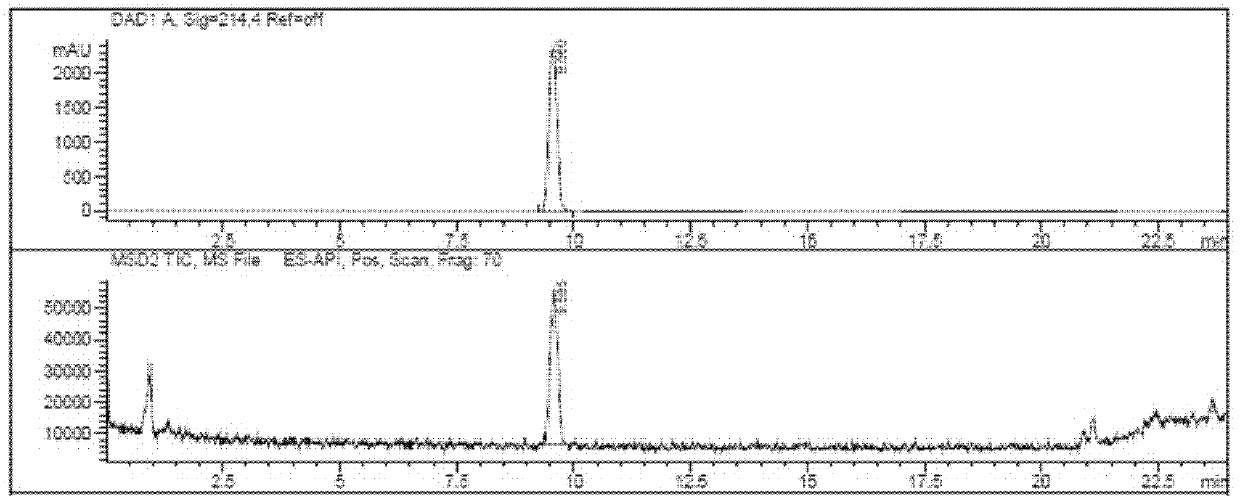
[0030]
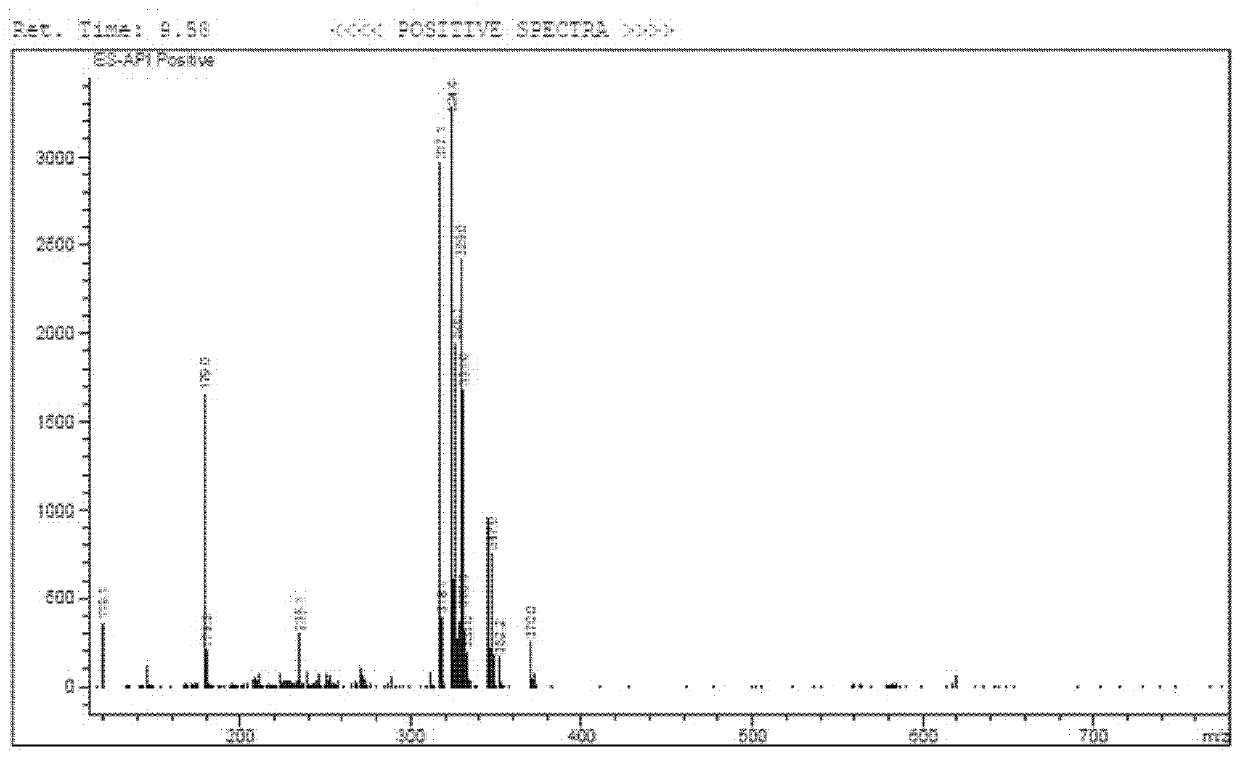
[0031] Electrospray mass spectrometry characterization data is: 306+23...
Embodiment 2
[0040] Synthesis of ammonium dichloroacetate
[0041] Step 2.1 Esterification of 2,2'-dichloroacetyl chloride with 9-fluorenylmethanol to obtain fluorenylmethyl dichloroacetate
[0042] Add 5.00 g of 9-fluorenylmethanol and 4.51 g of 2,2'-dichloroacetyl chloride to 20 ml of toluene solution, mix and heat to 110°C for 2 hours under reflux. After the reaction is detected by TLC, the reaction solution is placed in After vacuum concentration at 70°C, and then adding 10 ml of toluene to concentrate, a solid crude product was obtained.
[0043] The obtained above crude product was crystallized at 55°C in 50 ml of acetone and petroleum ether mixed solvent with a volume ratio of 1:9 to obtain 6.01 g of the formula The compound, fluorenylmethyl dichloroacetate. After concentrating and recrystallizing the mother liquor, 0.69g of formula The compound, the yield is 86%.
[0044]
[0045] Electrospray mass spectrometry characterization data is: 306+23 (Na + ), see ...
Embodiment 3
[0054] Synthesis of Calcium Dichloroacetate
[0055] Step 3.1 Esterification of 2,2'-dichloroacetyl chloride with 9-fluorenylmethanol to obtain fluorenylmethyl dichloroacetate
[0056] Add 5.00 g of 9-fluorenylmethanol and 4.51 g of 2,2'-dichloroacetyl chloride to 20 ml of toluene solution, mix and heat to 110°C for 2 hours under reflux. After the reaction is detected by TLC, the reaction solution is placed in After concentrated in vacuo at 70°C, and concentrated by adding 10 ml of toluene, a crude solid product was obtained.
[0057] The obtained above crude product was crystallized at 55°C in 50ml of a mixed solvent of acetone and petroleum ether with a volume ratio of 1:9 to obtain 6.01g of the formula The compound, fluorenylmethyl dichloroacetate. After concentrating and recrystallizing the mother liquor, 0.69g of formula The compound, the yield is 86%.
[0058]
[0059] Electrospray mass spectrometry characterization data is: 306+23 (Na + ), see ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com