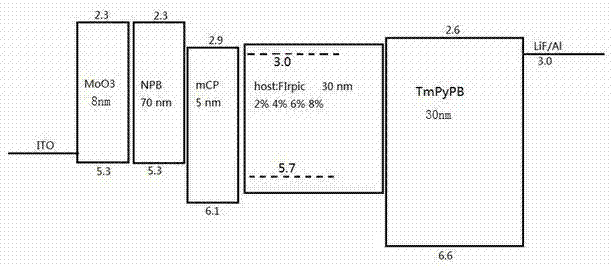
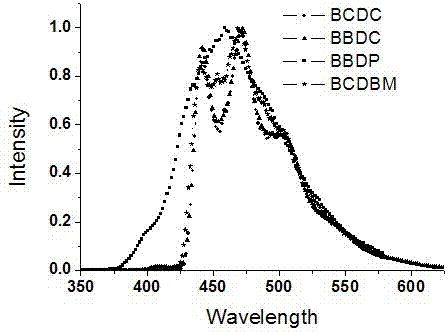
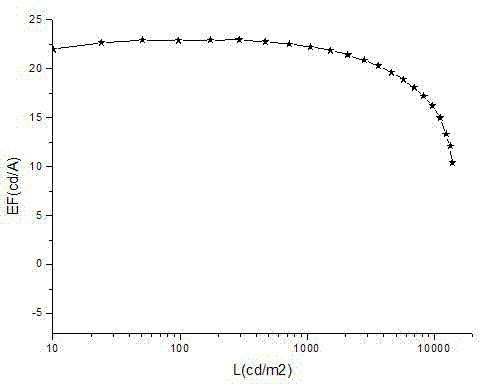
Fluorene-bridged blue phosphorescent main body material and preparation method and application thereof
A technology of triphenylamine fluorene and diphenylphosphine, which is used in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of hindering wide application, low glass transition temperature, and low material stability. , to achieve the effect of good hole and electron transport ability, high triplet energy, and increasing effective molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: when R in formula I 1 and R 2 is carbazolyl, R 3 and R 4 When it is phenyl, it is named as 9,9-diphenyl-3,6-dicarbazolylfluorene (BBDC), when R in formula I 1 and R 2 is carbazolyl, R 3 , R 4 When they are phenyl and 3-(9-phenylcarbazol) groups, they are named 9-phenyl-9-carbazolyl-3,6-dicarbazolylfluorene (BCDC), and their structural formulas are as follows:
[0043]
[0044] BBDCBCDC
[0045] The BBDC of the present invention can be synthesized by the following method.
[0046] Step 1: dissolving phenanthrenequinone and liquid bromine in nitrobenzene, heating to 80°C for 16 hours to obtain dibromophenanthrenequinone;
[0047] Step 2: dissolving dibromophenanthrenequinone, potassium hydroxide, and potassium permanganate in water, and reacting for 6 hours under reflux conditions to obtain dibromofluorenone;
[0048] Step 3: Dibromofluorenone and Benzene Grignard reagent were reacted in tetrahydrofuran at room temperature for 5 hours to obtain 9...
Embodiment 2
[0057] Embodiment 2: when R in formula I 1 and R 2 For diphenylphosphoryloxy, R 3 and R 4 When it is phenyl, it is named as 9,9-diphenyl-3,6-bis(diphenylphosphoryloxy)fluorene (BBDP); when R in formula I 1 and R 2 For diphenylphosphoryloxy, R 3 , R 4 When they are phenyl and 3-(9-phenylcarbazol) group respectively, they are named as 9-phenyl-9-carbazolyl-3,6-bis(diphenylphosphoryloxy)fluorene (BCDP), which The structural formula is as follows:
[0058]
[0059] BBDPBCDP
[0060] The BBDP of the present invention can be synthesized by the following method.
[0061] Step 1: dissolving phenanthrenequinone and liquid bromine in nitrobenzene, heating to 80°C for 16 hours to obtain dibromophenanthrenequinone;
[0062] Step 2: dissolving dibromophenanthrenequinone, potassium hydroxide, and potassium permanganate in water, and reacting for 6 hours under reflux conditions to obtain dibromofluorenone;
[0063] Step 3: Dibromofluorenone and Benzene Grignard reagent were reac...
Embodiment 3
[0072] Embodiment 3: when R in formula I 1 , R 2 For the second generation of tert-butyl substituted carbazolyl, R 3 , R 4 When they are phenyl and 3-(9-phenylcarbazolyl) respectively, they are named 9-phenyl-9-carbazolyl-3,6-di(di-tert-butylcarbazolyl)fluorene (BCDTC) , its structural formula is as follows:
[0073]
[0074] BCDTC
[0075] The above-mentioned BCDTC can be synthesized by the following method:
[0076] Step 1: dissolving phenanthrenequinone and liquid bromine in nitrobenzene, heating to 80°C for 16 hours to obtain dibromophenanthrenequinone;
[0077] Step 2: dissolving dibromophenanthrenequinone, potassium hydroxide, and potassium permanganate in water, and reacting for 6 hours under reflux conditions to obtain dibromofluorenone;
[0078] Step 3: Dibromofluorenone and Benzene Grignard reagent were reacted in tetrahydrofuran at room temperature for 5 hours to obtain 9-benzene-substituted dibromofluorene;
[0079] Step 4: Add an appropriate amount of tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com