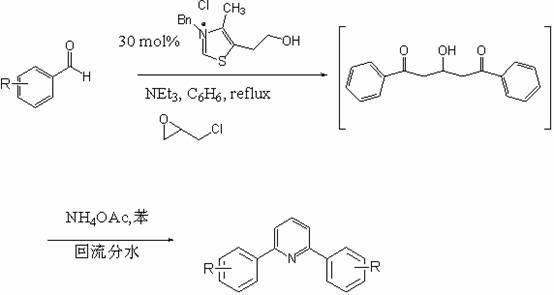
Synthetic method of diarylpyridines
A technology for diarylpyridines and a synthesis method, which is applied in the field of synthesis of similar compounds, can solve problems such as low yield and long reaction steps, and achieve the effects of improving synthesis efficiency, low preparation cost and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) 1 weight part of carbene catalyst 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazole chloride salt, 1 weight part of triethylamine), 100 weight parts of epichlorohydrin Add benzene into the container at room temperature and stir to react;
[0025] 2) Add 200 parts by weight of substituted aldehyde, heat and stir until the temperature of the mixture is 50°C, hold for 20 minutes or irradiate under microwave with a frequency of 150Hz for 5 minutes;
[0026] 3) Add 100 parts by weight of ammonium acetate and stir evenly;
[0027] 4) Heat and stir until the temperature of the mixture is 50°C, hold for 20 minutes or irradiate under microwave frequency of 150Hz for 5 minutes;
[0028] 5) After the reaction, benzene was evaporated to dryness, and the solid was recrystallized from ethanol to obtain diarylpyridine drugs.
Embodiment 2
[0031] 1) 2 parts by weight of carbene catalyst 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazole chloride, 2 parts by weight of triethylamine), 200 parts by weight of epichlorohydrin Add toluene into the container and stir at room temperature to react;
[0032] 2) Add 400 parts by weight of substituted aldehyde, heat and stir until the temperature of the mixture is 100°C, hold the temperature for 40 minutes or irradiate for 15 minutes under microwave with a frequency of 500Hz;
[0033] 3) Add 200 parts by weight of ammonium acetate and stir evenly;
[0034] 4) Heat and stir until the temperature of the mixture is 100°C, hold for 40 minutes or irradiate for 15 minutes under microwaves with a frequency of 150-500 Hz;
[0035] 5) After the reaction, the toluene was evaporated to dryness, and the solid was recrystallized from ethanol to obtain diarylpyridine drugs.
Embodiment 3
[0037] 1) 2 parts by weight of carbene catalyst 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazole chloride, 2 parts by weight of triethylamine), 200 parts by weight of epichlorohydrin Add xylene and stir in the container at room temperature to react;
[0038] 2) Add 400 parts by weight of substituted aldehyde, heat and stir until the temperature of the mixture is 80°C, hold for 30 minutes or irradiate under microwave with a frequency of 400Hz for 10 minutes;
[0039] 3) Add 200 parts by weight of ammonium acetate and stir evenly;
[0040] 4) Heat and stir until the temperature of the mixture is 80°C, hold for 30 minutes or irradiate for 12 minutes under microwave frequency of 150-400Hz;
[0041] 5) After the reaction, evaporate the xylene to dryness, and recrystallize the solid with ethanol to obtain diarylpyridine drugs.
[0042] Compound Identification:
[0043] 1,5-Diphenylpyridine: Melting point: 79-80°C, compared with literature (Wenkert, E.; Hanna, J.M.; Leftin, M. H.; Mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com