A method for monitoring the aggregation process of β-amyloid protein using aggregation-induced luminescence
A technique for aggregation-induced luminescence and amyloid protein, applied in the fields of biomedical research and clinical detection, to achieve the effects of improved sensitivity, low production cost, and improved detection speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 The aggregation process of β-amyloid protein 42 is monitored by fluorescent probe 1 coupled with maleimide, which is achieved by the following steps:
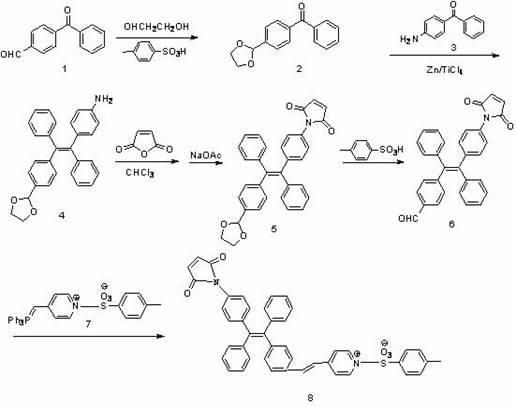
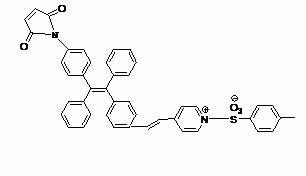
[0029] 1. Synthesis of fluorescent probe 1 coupled with maleimide, which is water-soluble. The synthetic route is shown below.
[0030] 2. Purchase Aβ42 whose glycine at position 2 is mutated into cysteine, and dissolve it in phosphate buffer solution (0.2 M, pH 7.4).
[0031] 3. Add maleimide-coupled fluorescent probe 1 in the above step 2, and use the specific covalent bond formed between the maleimide and the sulfhydryl group on cysteine to specifically bind the β-amyloid protein binding to aggregation-inducing fluorescent probes.
[0032]4. Using aggregation-induced luminescence enhancement to monitor the aggregation process of β-amyloid protein: put the prepared probes into a mixture containing 50 nM Aβ42 and 400 times complement C3, complement C4, IGA, IGM, IGG, IGD, IGE, fibroblast Cell growth facto...
Embodiment 2
[0036] Example 2 The aggregation process of β-amyloid protein 42 is monitored by fluorescent probe 2 coupled with lymide, which is achieved by the following steps:
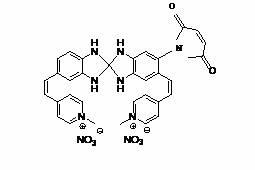
[0037] 1. Synthesis of fluorescent probe 2 coupled with maleimide, the probe is water-soluble. The synthetic route is shown below.
[0038] 2. Purchase Aβ42 whose glycine at position 2 is mutated into cysteine, and dissolve it in phosphate buffer solution (0.2 M, pH 7.4).
[0039] 3. In the above step 2, add a fluorescent probe 2 coupled with maleimide, and use the specific covalent bond formed between the maleimide and the sulfhydryl group on cysteine to convert the β-amyloid specific binding to aggregation-inducing fluorescent probes.
[0040] 4. Use the aggregation-induced luminescence enhancement method to monitor the aggregation process of β-amyloid protein: put the prepared probes into 30 nM Aβ42 and 300 times complement C3, complement C4, IGA, IGM, IGG, IGD, IGE, Fibroblast growth factor, epidermal gro...
Embodiment 3
[0044] Example 3 The aggregation process of β-amyloid protein 42 is monitored by fluorescent probe 3 coupled with maleimide, which is achieved by the following steps:
[0045] 1. Synthesis of maleimide-coupled fluorescent probe 3, which is water-soluble. The synthetic route is shown below.
[0046] 2. Purchase Aβ42 whose glycine at position 2 is mutated into cysteine, and dissolve it in phosphate buffer solution (0.2 M, pH 7.4).
[0047] 3. Add the maleimide-coupled fluorescent probe 3 in the above step 2, and use the specific covalent bond formed between the maleimide and the sulfhydryl group on the cysteine to specifically bind the amyloid beta protein binding to aggregation-inducing fluorescent probes.
[0048] 4. Use the aggregation-induced luminescence enhancement method to monitor the aggregation process of β-amyloid protein: put the prepared probes into the mixture containing 50 nM Aβ42 and 500 times complement C3, complement C4, IGA, IGM, IGG, IGD, IGE, fibroblast ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com