Granules containing dextromethorphan hydrobromide and guaiacol glycerin and preparation method thereof
A technology of dextromethorphan hydrobromide and guaiacol glycerol ether, which can be applied in the directions of ether/acetal active ingredients, bulk delivery, respiratory system diseases, etc. Drug concentration fluctuations, side effects, large fluctuations in blood drug concentration, and many times of administration, etc., to overcome low stability, avoid peak-to-valley phenomenon, and prolong action time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
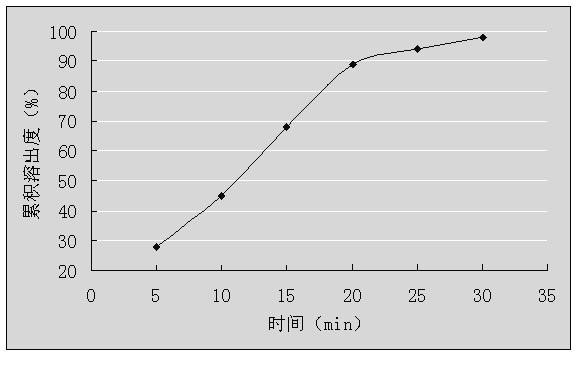
Embodiment 1
[0030] Granule prescription one:
[0031]
[0032] Note: Hydroxypropyl-β-cyclodextrin can also choose CAS128446-35-5 (molecular weight 1541.54); or CAS136019-12-0 (molecular weight 1541.54).
[0033] Preparation Process:
[0034] 1) Dry dextromethorphan hydrobromide, guaiacol glyceryl ether, hydroxypropyl-β-cyclodextrin, diluent and adhesive at 50°C for 3 hours, pass through a 100-mesh sieve for use;
[0035] 2) Make hydroxypropyl-β-cyclodextrin a saturated aqueous solution at 45°C, and then place them in a high-speed mixer, start stirring at a speed of 1000r / min;
[0036] 3) Dissolve dextromethorphan hydrobromide in 30% mass-volume ratio of acetonitrile aqueous solution, and then slowly add them dropwise to the saturated hydroxypropyl-β-cyclodextrin aqueous solution prepared in step 2) at a speed of 1 mL / min, continue to stir for 3 hours after the dropwise addition is completed, stop stirring, and spray dry to obtain cyclodextrin inclusion compound;
[0037] 4) Pass th...
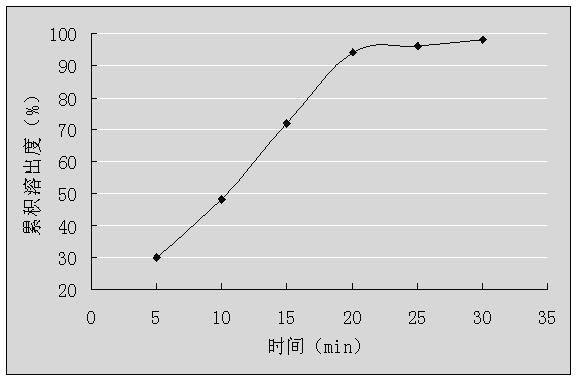
Embodiment 2
[0039] Granule prescription two:
[0040]
[0041] Note: Hydroxypropyl-β-cyclodextrin can also choose CAS128446-35-5 (molecular weight 1541.54); or CAS136019-12-0 (molecular weight 1541.54).
[0042] Preparation Process:
[0043] 1) Dry dextromethorphan hydrobromide, guaiacol glyceryl ether, hydroxypropyl-β-cyclodextrin, diluent and adhesive at 50°C for 3 hours, pass through a 100-mesh sieve for use;
[0044] 2) Prepare hydroxypropyl-β-cyclodextrin into saturated aqueous solutions at 60°C, and then place them in high-speed mixers, start stirring at a speed of 3000r / min;
[0045]3) Dissolve dextromethorphan hydrobromide in 30% mass-volume ratio of acetonitrile aqueous solution, and then slowly add them dropwise to the saturated hydroxypropyl-β-cyclodextrin aqueous solution prepared in step 2) and keep stirring, at a speed of 3 mL / min, continue to stir for 3 hours after the dropwise addition is completed, stop stirring, and spray dry to obtain cyclodextrin inclusion compou...
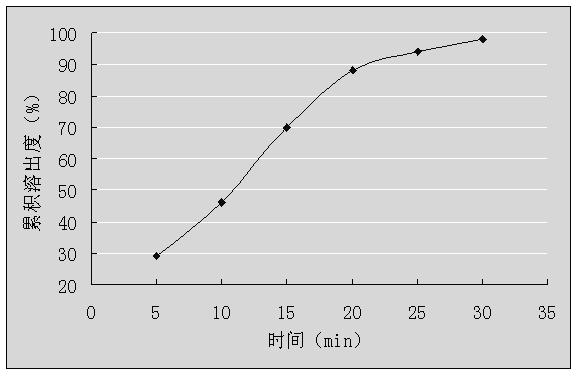
Embodiment 3
[0048] Granule prescription three:
[0049]
[0050] Note: Hydroxypropyl-β-cyclodextrin can also choose CAS128446-35-5 (molecular weight 1541.54); or CAS136019-12-0 (molecular weight 1541.54).
[0051] Preparation Process:
[0052] 1) Dry dextromethorphan hydrobromide, guaiacol glyceryl ether, hydroxypropyl-β-cyclodextrin, diluent and adhesive at 50°C for 3 hours, pass through a 100-mesh sieve for use;
[0053] 2) Prepare hydroxypropyl-β-cyclodextrin into saturated aqueous solutions at 50°C, and then place them in high-speed mixers, start stirring, and rotate at a speed of 750r / min;
[0054] 3) Dissolve dextromethorphan hydrobromide in 30% mass-volume ratio of acetonitrile aqueous solution, and then slowly add them dropwise to the saturated hydroxypropyl-β-cyclodextrin aqueous solution prepared in step 2) and keep stirring, at a speed of 3 mL / min, continue to stir for 3 hours after the dropwise addition is completed, stop stirring, and spray dry to obtain cyclodextrin in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com