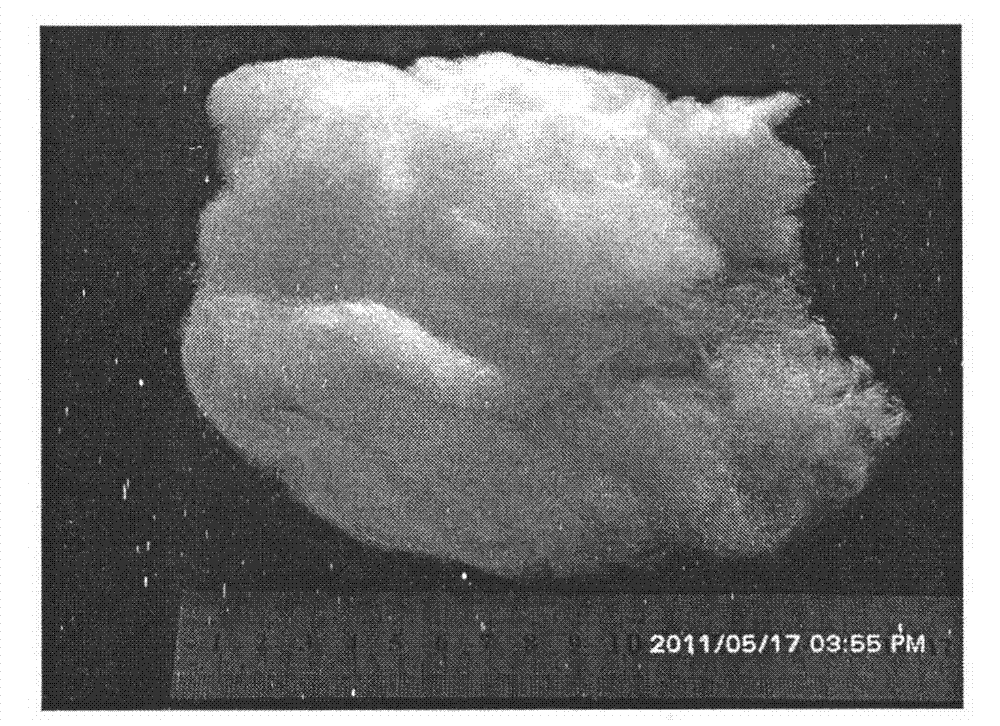
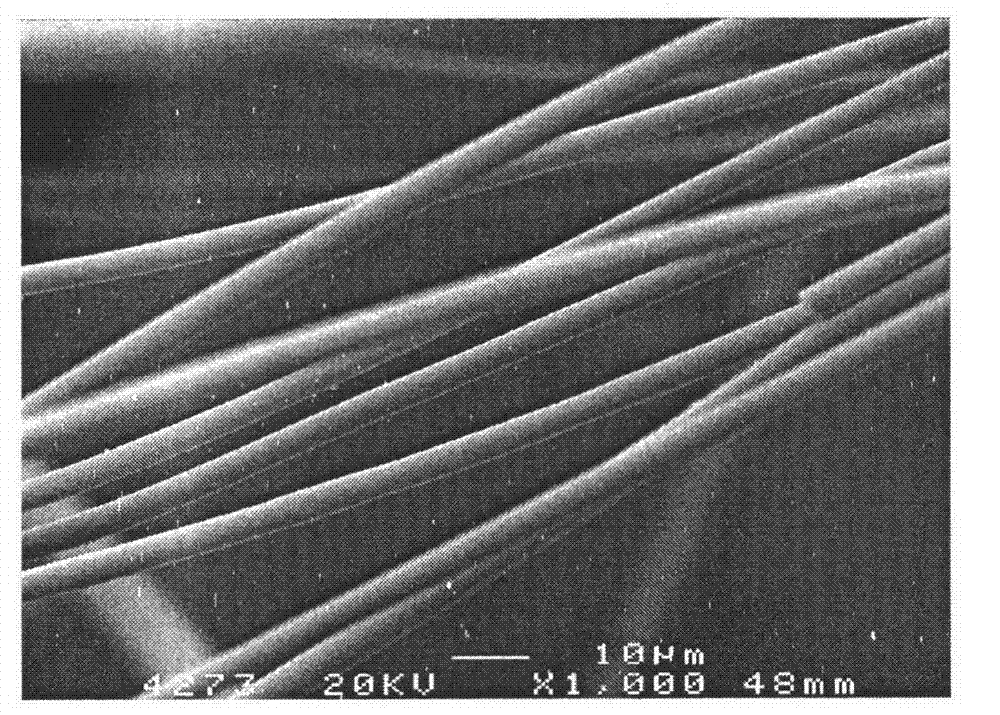
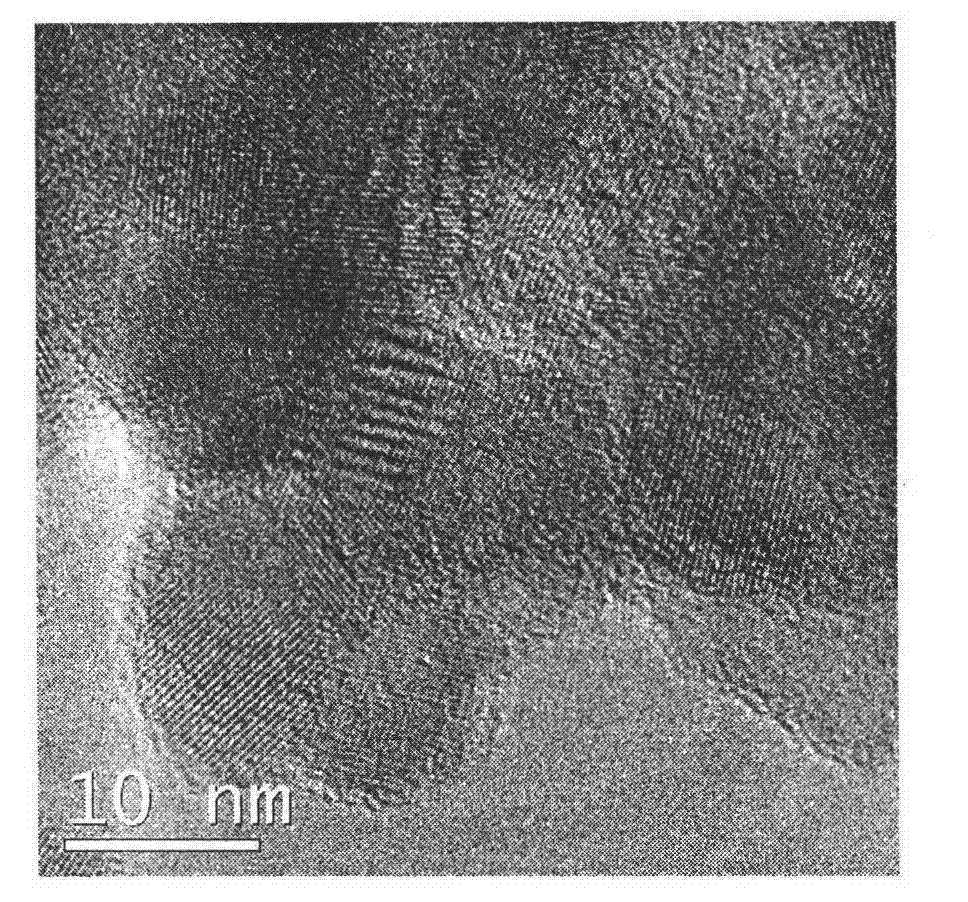
Preparation method of titanium dioxide fibers with photocatalysis function and polycrystalline nanostructure
A technology of titanium dioxide and polycrystalline structure, which is applied in the direction of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc., can solve the problems of many types of organic raw materials, difficulties in industrial scale-up, and complex glue-making processes, etc., to achieve improved photocatalysis Excellent performance, good spinnability, rich pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] Below to the TiO of the present invention 2 The preparation method of fiber is described in detail:
[0016] (1) prepare the filtrate that titanium acetate is dissolved in ethanol
[0017] According to the ratio of titanium tetrachloride: potassium acetate=1mol: 3mol~4mol, measure (weigh) two kinds of raw materials respectively; According to the ratio of titanium tetrachloride: ethanol=1mL: 3mL~4mL, the tetrachloride Titanium is diluted in ethanol (measurement and dilution of titanium tetrachloride must be carried out in a fume hood), and the resulting solution is recorded as liquid A; according to the ratio of potassium acetate: ethanol = 1g: 1mL ~ 2mL, dilute potassium acetate to ethanol , and continue to stir for 0.5h to 1h to fully dissolve potassium acetate, and the resulting solution is recorded as liquid B; at 0°C to 10°C and under stirring conditions, add the above solution B dropwise to solution A (or add A dropwise to In B) carry out mixed reaction to genera...
Embodiment 1
[0028] (1) prepare the filtrate that titanium acetate is dissolved in ethanol
[0029] According to the ratio of titanium tetrachloride:potassium acetate=1mol:4mol, 33mL of titanium tetrachloride and 118g of potassium acetate were weighed. According to the ratio of titanium tetrachloride: ethanol = 1mL: 4mL, titanium tetrachloride was diluted in 132mL ethanol, and the resulting solution was recorded as liquid A; according to the ratio of potassium acetate: ethanol = 1g: 2mL, potassium acetate was diluted in 236mL In ethanol, stir for 0.5 h to fully dissolve potassium acetate, and the resulting solution is designated as liquid B. Under the condition of stirring at 0°C, add liquid B dropwise to liquid A for a mixed reaction to generate titanium acetate and potassium chloride, and continue stirring for 1 hour after the drop to complete the reaction. Then, use the property that potassium chloride is insoluble in ethanol It was removed by filtration to obtain a clear titanium acet...
Embodiment 2
[0037] (1) prepare the filtrate that titanium acetate is dissolved in ethanol
[0038] According to the ratio of titanium tetrachloride:potassium acetate=1mol:3mol, 33mL of titanium tetrachloride and 89g of potassium acetate were weighed. According to the ratio of titanium tetrachloride: ethanol = 1mL: 3mL, titanium tetrachloride was diluted in 99mL ethanol, and the resulting solution was recorded as liquid A; according to the ratio of potassium acetate: ethanol = 1g: 1mL, potassium acetate was diluted in 89mL In ethanol, stir for 1 h to fully dissolve potassium acetate, and the resulting solution is designated as liquid B. Then, under stirring condition at 10°C, liquid B was added dropwise to liquid A for a mixed reaction to generate titanium acetate and potassium chloride. After the dropping was completed, stirring was continued for 0.5 h to complete the reaction. Then, potassium chloride was insoluble in ethanol It is removed by filtration to obtain a clear titanium acetat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com