Catalyst and preparation method for benzene hydroxylation to phenol
A benzene hydroxylation and catalyst technology, which is applied in the field of heteropoly acid catalyst and preparation, can solve the problems of difficult catalyst recovery, low benzene conversion rate and phenol yield, and complicated catalyst preparation process, so as to avoid deep oxidation and improve benzene Conversion rate, effect of promoting reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
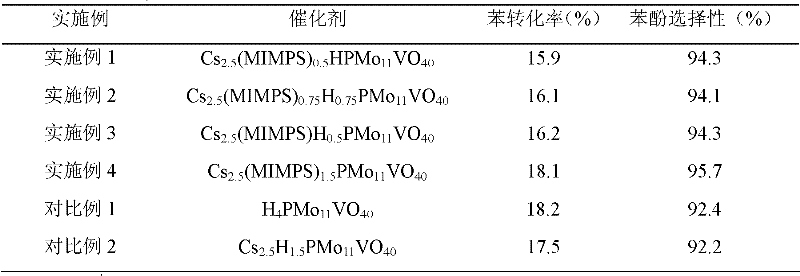
[0024] Cs 2.5 (MIMPS) 0.5 HPMo 11VO 40 The preparation and catalytic performance of
[0025] h 4 PMo 11 VO 40 Preparation: Weigh 0.11mol of MoO 3 and 0.005mol of V 2 o 5 were dissolved in water respectively, then the two solutions were mixed and heated to 373K, and 0.01 mol of H was added under stirring 3 PO 4 , and the suspension was reacted at 373K for 24h, and after filtration, the obtained solution was prepared into a 500mL standard solution, and the concentration of the solution was 0.02mol L -1 .
[0026] Cs 2.5 h 1.5 PMo 11 VO 40 Preparation: Weigh 0.005mol of Cs 2 CO 3 Dissolve in water to form a solution, drop by drop into the previously pipetted H 4 PMo 11 VO 40 In 200mL standard solution, heat to 373K, react for 5-7h, centrifuge the obtained suspension, pour the supernatant and dry it in a blast oven at 100°C to obtain Cs 2.5 h 1.5 PMo 11 VO 40 .
[0027] Cs 2.5 (MIMPS) 0.5 HPMo 11 VO 40 Preparation: Weigh the prepared Cs 2.5 h 1.5 PMo...
Embodiment 2
[0030] Cs 2.5 (MIMPS) 0.75 h 0.75 PMo 11 VO 40 The preparation and catalytic performance of
[0031] The preparation method is the same as that of Example 1, except that the amount of MIMPS added is 0.15 mmol.
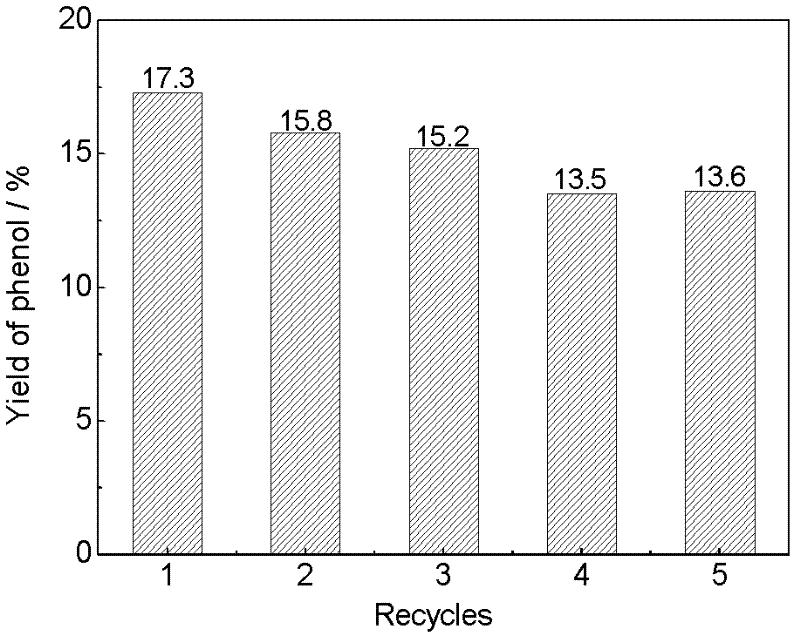
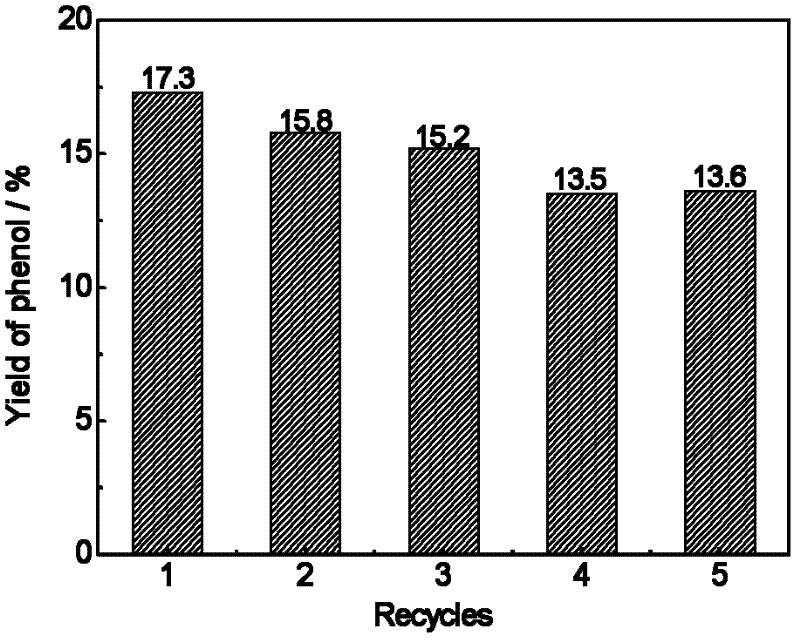
[0032] Weighed 0.05mmol (ie 0.113g) solid catalyst and applied it to the liquid phase oxidation reaction of benzene, the results are shown in Table 1.
Embodiment 3
[0034] Cs 2.5 (MIMPS)H 0.5 PMo 11 VO 40 The preparation and catalytic performance of
[0035] The preparation method is the same as in Example 1, except that the amount of MIMPS added is 0.2 mmol.
[0036] Weighed 0.05mmol (ie 0.116g) solid catalyst and applied it to the liquid phase oxidation reaction of benzene, the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com