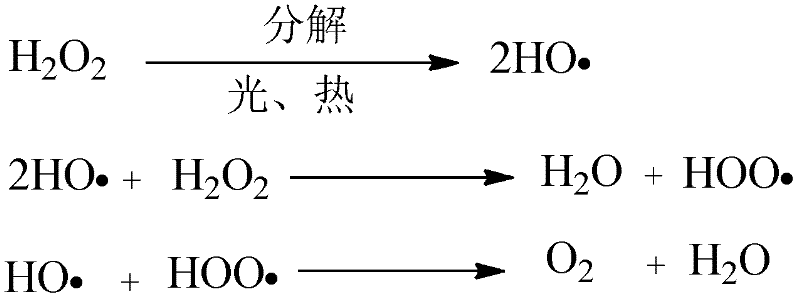
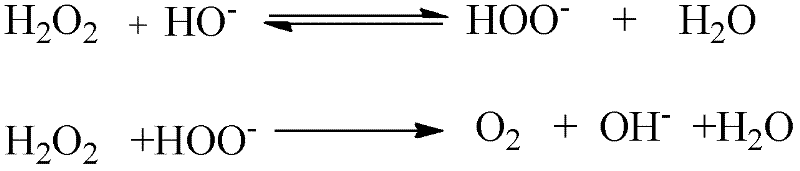
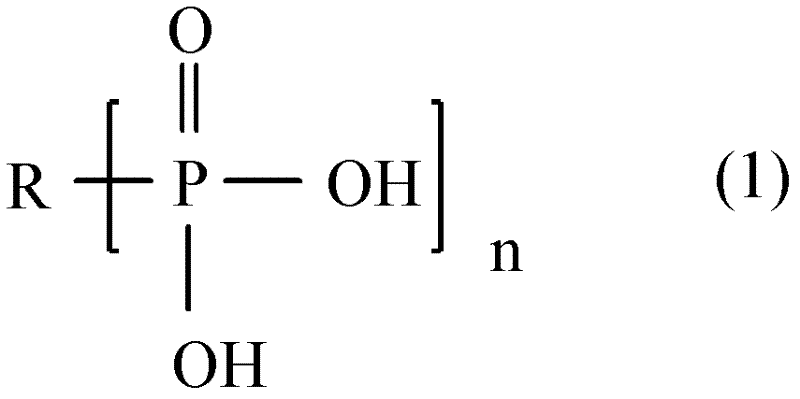A hydrogen peroxide stabilizer
A hydrogen peroxide and stabilizer technology, applied in the direction of peroxide/peroxy hydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, chemical instruments and methods, etc., can solve the problem of unsuitable strong alkali Use under certain conditions, not suitable for storage and application of hydrogen peroxide, etc., to achieve the effects of improved safety, simple and convenient use, and reduced decomposition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Design a kind of stabilizing agent of hydrogen peroxide, be used under strongly alkaline condition, be made up of following mass percent raw material:
[0040] Hydroxyethylene Diphosphonic Acid 30%,
[0041] Magnesium Chloride 10%,
[0042] Distilled water 60%.
[0043] The preparation method is as follows: adding hydroxyethylene diphosphonic acid into magnesium chloride according to the stated ratio, mixing evenly, then adding distilled water to reach the ratio, and stirring for 0.5 hours to obtain the hydrogen peroxide stabilizer.
[0044] After inspection, when the alkali concentration in the hydrogen peroxide (carry out mole calculation with KOH) is 11mol / L, temperature is 0 ℃, when the addition of gained stabilizing agent is 1%, the stability of hydrogen peroxide reaches 99.1%.
Embodiment 2
[0046] Design a kind of stabilizing agent of hydrogen peroxide, be used under strongly alkaline condition, be made up of following mass percent raw material:
[0047] Hydroxyethylene diphosphonic acid 29%,
[0049] Distilled water 58%.
[0050] The preparation method is as follows: adding hydroxyethylene diphosphonic acid into magnesium sulfate according to the stated proportion, mixing evenly, then adding distilled water to reach the proportion, and stirring for 1.5 hours to obtain the hydrogen peroxide stabilizer.
[0051] After inspection, when the alkali concentration in the hydrogen peroxide (carry out mole calculation with KOH) is 12mol / L, temperature is-5 ℃, when the addition of stabilizing agent is 1%, the stability of hydrogen peroxide reaches 99.2%.
Embodiment 3
[0053] Design a kind of stabilizing agent of hydrogen peroxide, be used under strongly alkaline condition, be made up of following mass percent raw material:
[0054] Methylene-1,1-diphosphonic acid 30%,
[0055] Magnesium Acetate 10%,
[0056] Distilled water 60%.
[0057]Its preparation method is: add methylene-1,1-diphosphonic acid into magnesium acetate according to the stated ratio, mix evenly, then add distilled water to reach the ratio, and stir for 3 hours to obtain the hydrogen peroxide stabilizer .
[0058] After testing, when the alkali concentration in the hydrogen peroxide (carry out molar calculation with KOH) is 14mol / L, temperature is-10 ℃, when the addition of gained stabilizing agent is 1%, the stability of hydrogen peroxide reaches 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com