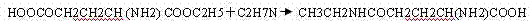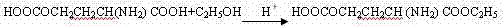The method of synthesizing theanine
A technology of theanine and glutamic acid, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problems of affecting the quality of L-theanine, danger to life and environment, and many types of products. , to achieve the effect of low cost, high equipment requirements and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Add ethanol to 1300L in a 1500L stainless steel reaction tank, add L-glutamic acid (HOOCOCH 2 CH 2 CH (NH 2 ) COOH) 300kg (N mol), cool the ice water to 0-5°C, add sulfuric acid 200kg (2N.mol), remove the ice water after the dropwise addition, control the temperature at 8-10°C, and react for 8-10 hours, the reaction formula is as follows :
[0031]
[0032] After the TLC monitoring is qualified, it is neutralized to pH 7.0-7.5 by passing through ethylamine, centrifuged, washed with ethanol until the centrifugate is colorless, and L-glutamic acid-5-ethyl ester (HOOCOCH) containing 20% ethanol is obtained. 2 CH 2 CH (NH 2 ) COOC 2 h 5 )350kg. (Mother liquor can recover ethanol and detect and be used for this step reaction of next batch)
[0033] 2. Add 500L of ethanol to a 1500L stainless steel reverse tank, add 180kg of L-glutamic acid-5-ethyl ester under stirring, and add 70% ethylamine (C 2 h 7 N) 600L of aqueous solution, reacted at 20-25°C for 72 ho...
Embodiment 2
[0038] 1. Add ethanol to 1100L in a 1500L stainless steel reaction tank, add 300kg (N mol) of L-glutamic acid, cool down to 0-5°C with ice water, add 280kg (2N.mol) of sulfuric acid, and remove the ice water after the dropwise addition , control the temperature at 8-10°C, react for 8-10 hours, after TLC monitoring is qualified, pass through ethylamine to neutralize to pH 7.0-7.5, centrifuge, wash with ethanol until the centrifuge is colorless, and obtain 20% ethanol L-glutamic acid-5-ethyl ester 370kg.
[0039] 2. Add 600L of ethanol to a 1500L stainless steel reverse tank, add 175kg of L-glutamic acid-5-ethyl ester under stirring, add about 550L of 70% ethylamine under cooling, and react at 20-25°C for 48 hours until it is basically complete, and recover Excess ethylamine (recovered ethylamine can be used in the next batch of this step reaction) to obtain L-theanine ethylamine solution, recover ethylamine and ethanol, after concentration, add ethanol to precipitate crystals, ...
Embodiment 3
[0042] 1. get aforementioned embodiment one, the mother liquor that centrifuges gained in the second step of two is about 150L of feed liquid after reclaiming the ethanol concentration, add the ethanol 300L that reclaims, add aforementioned embodiment one, the second step that reclaims in two Amine 500L (contains a small amount of ethanol when recovered), reacted at 20-25°C for 48 hours until almost complete, recovered excess ethylamine (recovered ethylamine can be used in the next batch of this step reaction) to obtain L-theanine ethylamine solution of about 750L, after concentration, add ethanol to precipitate crystals, and centrifuge to obtain 80KG crude L-theanine. (The centrifuged mother liquor is to be detected after recovery of ethylamine and ethanol).
[0043] 2. Add 80KG of L-theanine crude product to 280L water, heat up and dissolve, add 1KG activated carbon for decolorization and filtration, add 95% edible ethanol twice (V / V), precipitate crystals, centrifuge to sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com