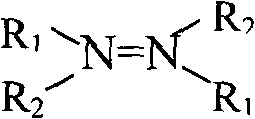A new method for preparing biuret by utilizing ketazine
A technology for biuret and ketazine, which is applied in the field of preparing biuret by using ketazine, can solve the problems of environmental pollution damage, technical difficulty, long reaction time and the like, and achieves shortened reaction time, high product purity, The effect of fast heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take a 1L three-necked flask, add 200mL of acetone azine, prepare 500mL of 1+1 sulfuric acid, take 160mL of sulfuric acid solution, add it dropwise to the three-necked flask, set the reaction temperature to 60°C, and weigh 173g after the addition of sulfuric acid is completed. Add urea into the three-necked flask at one time, and the temperature of the oil bath increases rapidly. When the temperature rises to 105°C, keep stirring at this temperature, and a white precipitate will appear in about ten minutes. Continue stirring for 2 hours, filter, and wash with water twice. , the filtrate was distilled with ammonia, the white precipitate was put into an oven, and baked at 50° C. for 8 hours. The measured melting point was 248° C., and the white substance was determined to be biurea, with a yield of 85%.
Embodiment 2
[0022] Take a 1L three-neck flask, add 200mL butanazine, configure 1+2 sulfuric acid 500mL, take 240mL sulfuric acid solution, add it dropwise to the three-necked flask, set the reaction temperature to 70°C, wait until the sulfuric acid is added dropwise, weigh Add 173g of urea into the three-necked flask at one time, and the temperature of the oil bath increases rapidly. When the temperature rises to 110°C, keep stirring at this temperature, and a white precipitate will appear in about five minutes. Continue stirring for 2 hours, filter, and wash with water for two hours. The second time, the filtrate was distilled with ammonia, and the white precipitate was put into an oven, and baked at 50°C for 8 hours. The melting point was 248°C. The white substance was determined to be biurea, and the yield was 86%.
Embodiment 3
[0024] Take a 1L three-necked flask, add 200mL of acetonazine, configure 500mL of 1+1 hydrochloric acid, take 260mL of hydrochloric acid solution, add it dropwise to the three-necked flask, set the reaction temperature to 80°C, and weigh 173g after the addition of hydrochloric acid is completed. Add urea into the three-necked flask at one time, and the temperature of the oil bath increases rapidly. When the temperature rises to 112°C, a white precipitate is formed. Continue to heat up to 120°C, and keep stirring at this temperature for 1 hour, filter, and wash with water twice. , the filtrate was distilled with ammonia, the white precipitate was put into an oven, baked at 50°C for 8h, and its melting point was 248°C. It was determined that the white substance was biurea, and the yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com