Method for preparing 5-substituted thiophenyl-benzimidazol-2-N-methoxycarbonyl compound
A -2-N-, methoxycarbonyl technology, applied in the field of preparation of phenylthio-benzimidazole-2-N-methoxycarbonyl compounds, can solve the problem of lack of market competitiveness, high risk, High price and other problems, to achieve the effect of easy procurement and preservation, low price and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
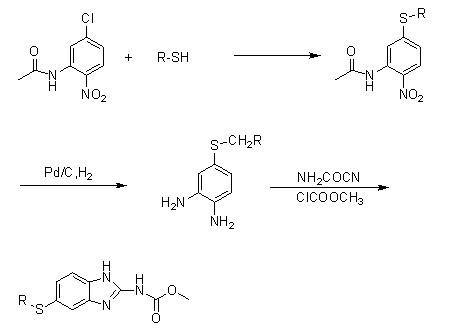
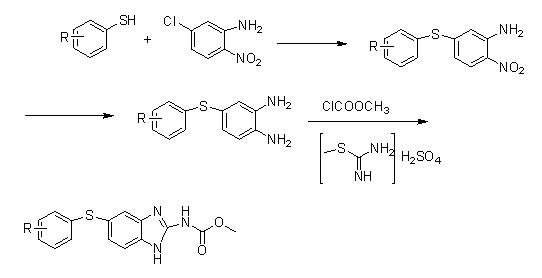
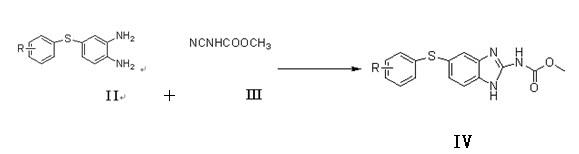
[0033] Synthesis of Compound IA (R=H):
[0034] 220 g of thiophenol (2.0 mol), 358.8 g of anhydrous potassium carbonate (2.6 mol) and 345 g of 5-chloro-2-nitroaniline (2.0 mol) were added to 2500 ml of N,N-dimethylformamide , reflux reaction for 3 hours, concentrated under reduced pressure and evaporated 1800 milliliters of solvent, the residue was poured into 3000 milliliters of ice water, stirred for 45 minutes, filtered, the filter cake was washed with 1000 milliliters of water, and dried to obtain 468.3 grams of off-white powder.
[0035] Yield: 95.18% (calculated as thiophenol).
[0036] Synthesis of Compound IIA (R=H):
[0037] Add 246 grams of compound I (1.0mol) and 146.3 grams of ammonium chloride (2.5mol) into 1000 milliliters of anhydrous methanol, stir, and under ice-salt bath cooling, add 43.8 grams of sodium borohydride (1.15mol) in batches, and the addition is complete , slowly returned to room temperature, stirred and reacted for 2 hours, after the reacti...
Embodiment 2
[0046] Synthesis of Compound IB (R=p-Cl):
[0047] 144.5 grams of 4-chlorothiophenol (1.0mol), 165.6 grams of anhydrous potassium carbonate and 172.5 grams of 5-chloro-2-nitroaniline were added to 1500 milliliters of N, N-dimethylformamide, refluxed for 5 hours, After the reaction was completed, 1000 ml of solvent was evaporated by concentration, and the residue was added to 1600 ml of ice water, stirred for 45 minutes, filtered, and the filter cake was washed with 800 ml of water and dried to obtain 236.8 g of light yellow solid powder.
[0048] Yield: 84.42% (calculated as 4-chlorothiophenol).
[0049] Synthesis of Compound IIB (R=p-Cl):
[0050]Add 280.5 grams of compound IB and 163.8 grams of ammonium chloride (2.8 mol) into 1200 ml of methanol, stir, cool to -5°C, add 47.5 grams of sodium borohydride (1.25 mol) in batches, after the addition is complete, return to room temperature and stir the reaction After 3 hours, after the reaction was over, the reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com