Ganciclovir tablet composition and its preparation method
A technology of ganciclovir hydrate and lovir tablet, which is applied in the field of ganciclovir tablet composition, can solve the problems of difficult quality standard control, high packaging and storage costs, increased mixing difficulty, etc. Improve bioavailability, accurate dosage and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of ganciclovir hydrate crystals:
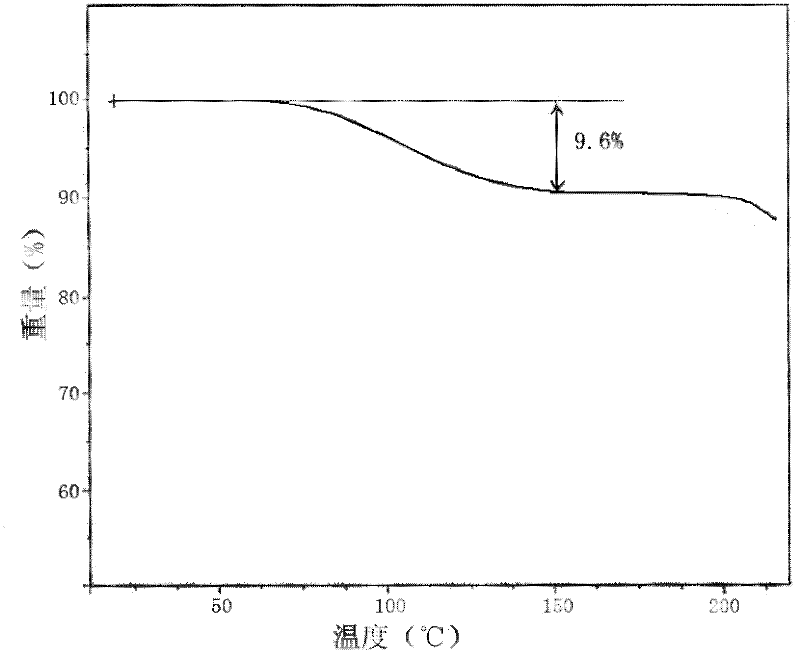
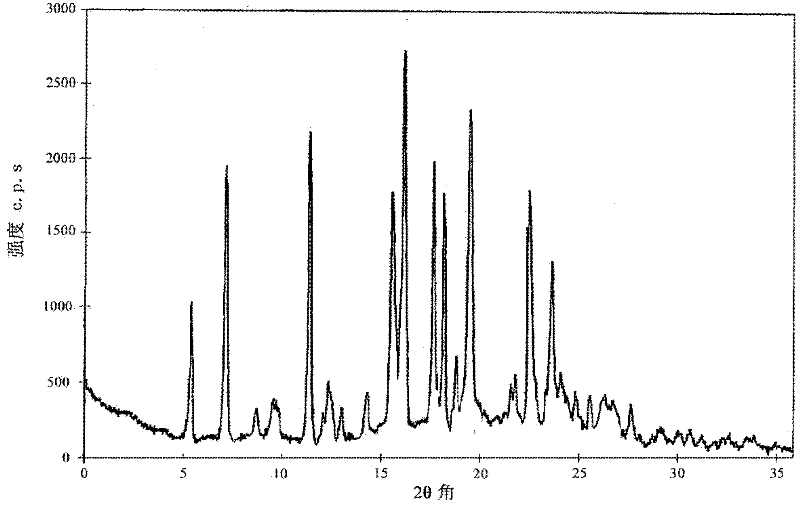
[0029] At a temperature of 65°C, 10g of ganciclovir was dissolved in 90ml of acetic acid-water mixed solvent (the volume ratio of acetic acid to water was 3 / 2), and the ganciclovir solution was slowly cooled to -5°C to precipitate crystals, and then Stand at 5°C for 6 hours, filter, and wash the filter cake with acetone three times to obtain ganciclovir hydrate crystals. Thermogravimetric analysis showed that ( figure 1 Shown), the content of the water of crystallization that contains in the ganciclovir crystal that obtains is 9.60% (theory is 9.57%), promptly contains 1.5 water of crystallization, the X-ray powder diffraction of gained ganciclovir hydrate crystal ( figure 2 Shown) the characteristic peaks are displayed at 5.46°, 7.25°, 11.45°, 15.62°, 16.21°, 17.64°, 18.15°, 19.54°, 22.55° and 23.71° diffraction angles.
Embodiment 2
[0031] Preparation of ganciclovir hydrate crystals:
[0032] Under the condition that the temperature is 70°C, 10g of ganciclovir is dissolved in 85ml of acetic acid-water mixed solvent (the volume ratio of acetic acid to water is 2 / 1), and the temperature of the ganciclovir solution is slowly cooled to -5°C to precipitate The crystals were left to stand at 5° C. for 7 hours, filtered, and the filter cake was washed with acetone for 3 times to obtain ganciclovir hydrate crystals. Show through powder XRD detector and thermogravimetric analysis, accord with the result shown in the accompanying drawing of embodiment 1 product.
Embodiment 3
[0034] Preparation of ganciclovir hydrate crystals:
[0035] Under the condition that the temperature is 60°C, 10g of ganciclovir is dissolved in 90ml of acetic acid-water mixed solvent (the volume ratio of acetic acid to water is 5 / 3), the temperature of the ganciclovir solution is slowly cooled to 0°C, and crystals are precipitated , and then stood at 0° C. for 7 hours, filtered, and the filter cake was washed twice with acetone to obtain ganciclovir hydrate crystals. Show through powder XRD detector and thermogravimetric analysis, accord with the result shown in the accompanying drawing of embodiment 1 product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com