Low-field high-relaxation rate magnetic resonance imaging (MRI) contrast agent and preparation method
A technology of contrast agent and fullerene carboxylic acid, which is applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agent, etc., can solve the problems of poor targeting and low efficiency, and achieve the effect of improving relaxation efficiency and overcoming high technical cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
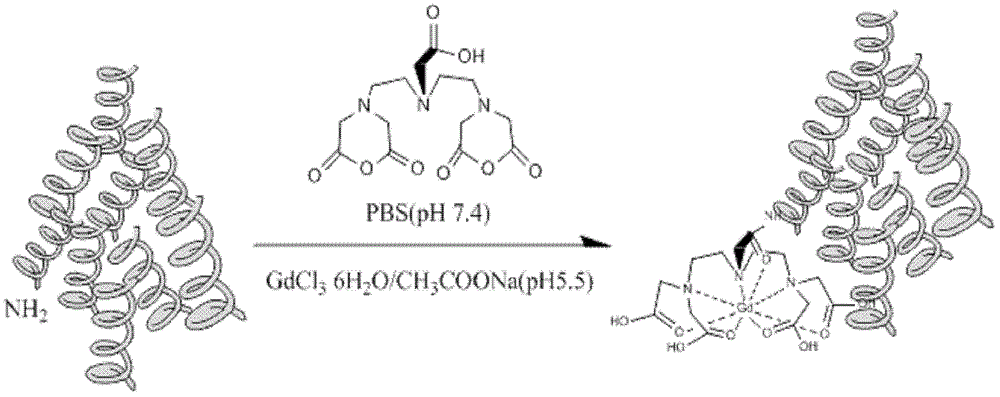
[0038] Embodiment 1: Preparation of Gd-DTPA-HSA macromolecular contrast agent
[0039] a) Dissolve 100mg HSA in 5mL PBS (pH 7.4) buffer solution, directly add cDTPA bisanhydride solid (cDTPA:HSA=200:1, molar ratio) in batches, use 0.1M NaOH solution to adjust pH=7, at room temperature Reaction 2h;
[0040] b) Separation and purification of the system obtained after the reaction by Sephadex G-25 (PD-10 mesh) with Sephadex, eluent: 0.5M CH 3 COONa (pH=6.0), was monitored with a UV-Vis spectrophotometer at 280nm to obtain a HSA-DTPA solution;
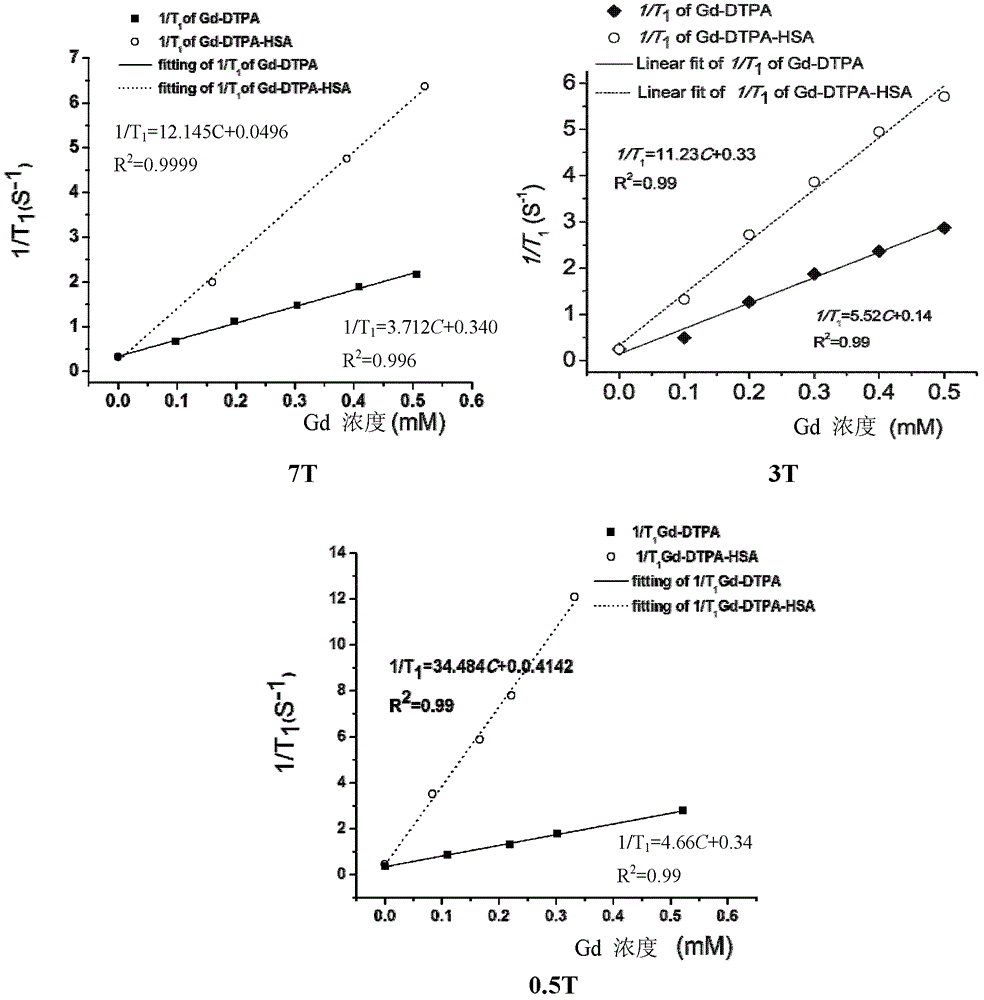
[0041] c) 112mg GdCl 3 ·6H 2 O(GdCl 3 ·6H 2 (O:HSA=200:1, molar ratio) was dissolved in 1 mL CH 3 COONa (pH=6.0, 0.5M) buffer solution was added to the HSA-DTPA solution, stirred at room temperature for 2 hours, and the small molecules were removed by dialysis using a Mw=12,000-14,000 dialysis bag, and then unreacted was removed by an ultrafiltration centrifuge tube with Mw=10,000 substances, monitored by measuring the conductivity ...
Embodiment 2
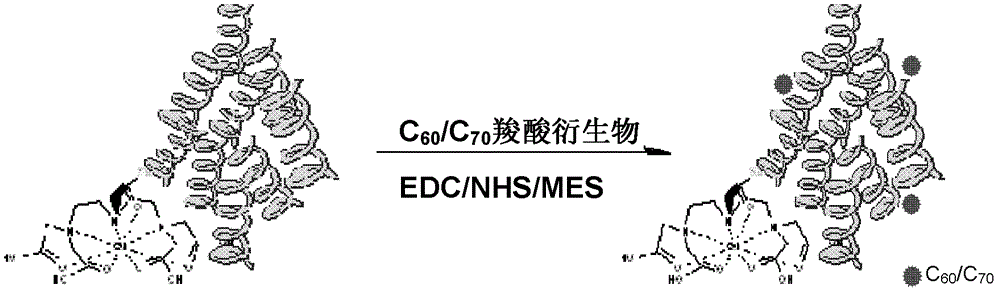
[0049] Embodiment 2: Preparation of conjugates of Gd-DTPA-HSA and fullerene carboxylic acid derivatives
[0050] a)(C 60 [C(COOH) 2 ] x (x=3)) Preparation: Reference (Bingel, C., Cyclopropanierung vonFullerenen. Chemische Berichte 1993, 126(8), 1957-1959.) Preparation of fullerene carboxylate derivatives followed by hydrolysis using NaH to give fullerene carboxylic acid derivatives.
[0051] b) 10mg fullerene carboxylic acid derivative (C 60 [C(COOH) 2 ] x (x=3)) was dissolved in 10mL MES (pH 5.5) buffer, added 10mg EDC and 5mg NHS (C 60 [C(COOH) 2 ] x With EDC, NHS molar ratio is 1:6:6), reaction at room temperature for 2h, to obtain fullerene carboxylic acid active ester.
[0052] c) Add 10 mg of Gd-DTPA-HSA to the above-mentioned fullerene carboxylic acid active ester solution, stir overnight at room temperature, use a Mw=12,000-14,000 dialysis bag to remove small molecules, and then use a Mw=10,000 ultrafiltration centrifuge tube Remove unreacted fullerene molecu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com