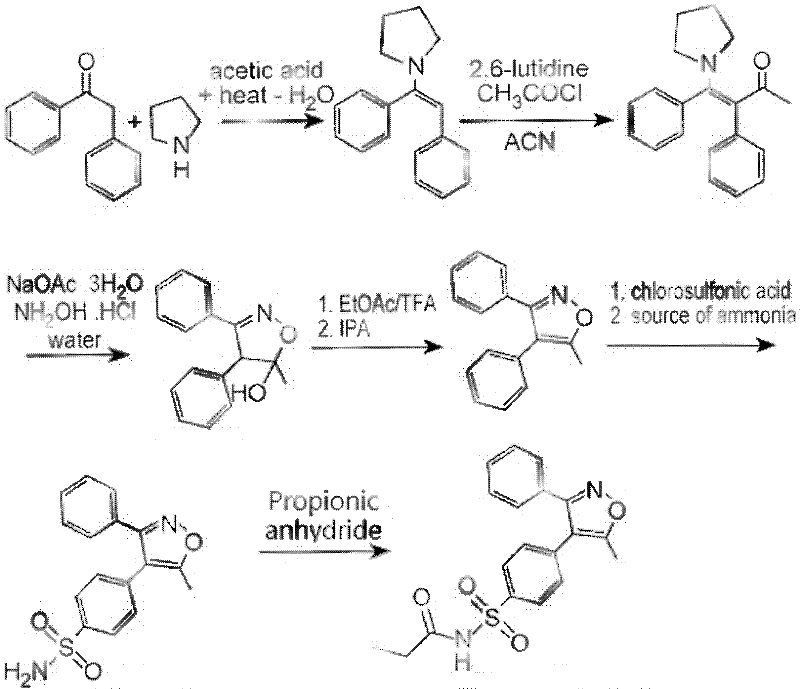
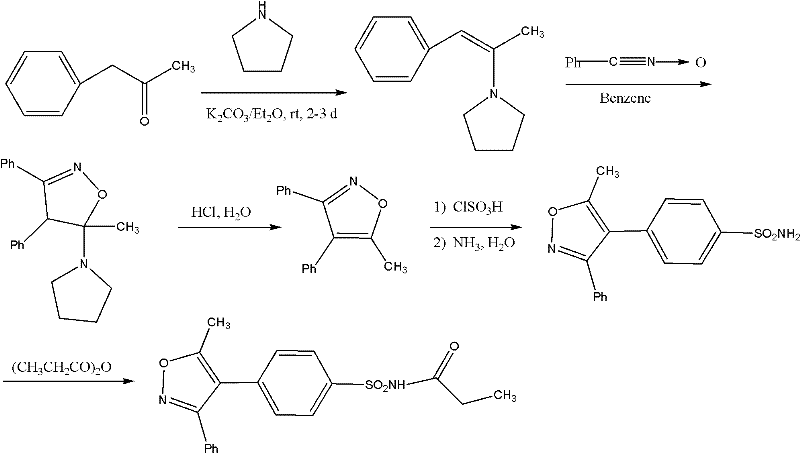
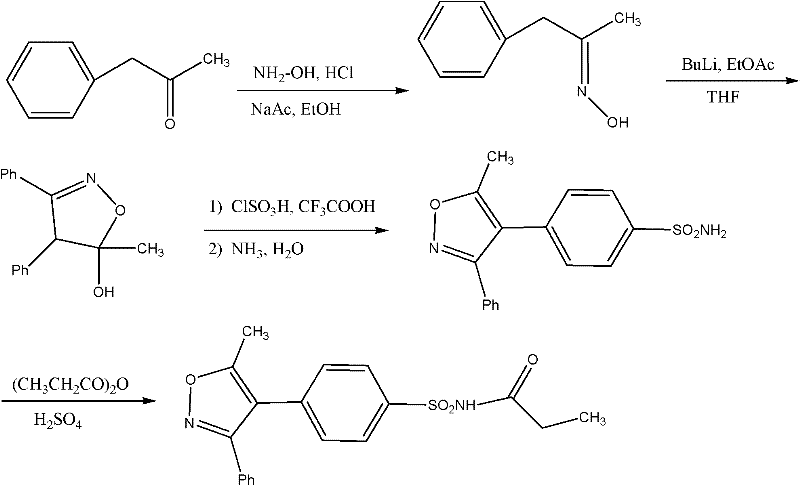
Method for preparing Parecoxib
A technology of parecoxib and valdecoxib, applied in the field of prodrugs of valdecoxib, can solve problems such as unfavorable safe production, increased difficulty in industrialized operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be further described below in conjunction with preferred embodiment, but the present invention is by no means limited to following embodiment.
[0026] The 1,2-benzophenone used in the present invention is purchased from ACROS Company of the United States, and other raw materials are analytically pure or chemically pure provided by Sinopharm Group.
[0027] 1. Preparation of 1-phenyl-2-(4-sulfophenyl)ethanone (5)
[0028] 19.6 g (0.1 mol) of 1,2-benzophenone was dissolved in 200 mL of acetone, and 11.6 g (0.1 mol) of chlorosulfonic acid was slowly added dropwise at 0°C. After the dropwise addition, the temperature was naturally raised to 25° C., and the reaction was continued for 5 h. After the reaction is complete, distill off the acetone, add 100mL of water to the residue and continue to reflux for 12h, after cooling, add 50mL of ethyl acetate for extraction, combine the ester layer, dry the ester layer with anhydrous sodium sulfate, filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com