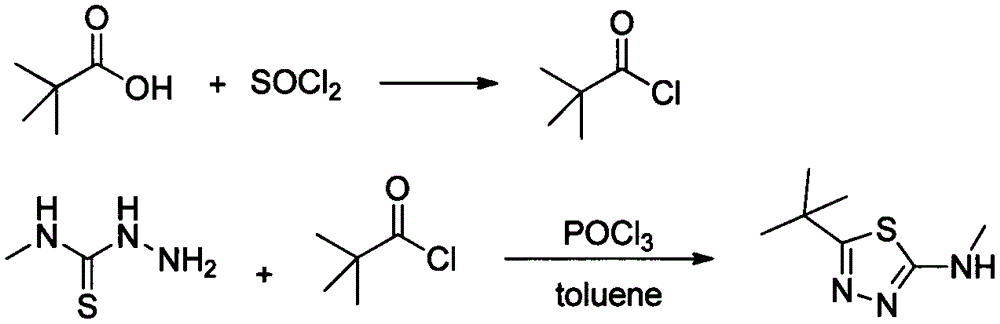
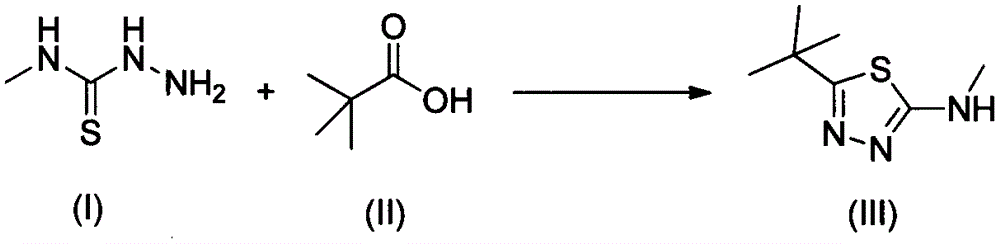
Preparation method of 2-methylamino-5-t-butyl-1,3,4-thiadiazole
A technology of methylamino and methylthiosemicarbazide, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, environmental pollution, and many by-products, and achieve the goal of less harsh reaction conditions, clean production process, and continuous operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 30g of pivalic acid into a 250mL three-necked flask, add 30mL of toluene to dissolve, raise the temperature to 80°C, and add 43g of solid phosgene in batches. After the addition was complete, the reaction was continued for 2 hours, and the released HCl gas was passed into water. Cool down to room temperature and set aside.
[0028] Add 28g of methylthiosemicarbazide and 30mL of toluene into a 500mL four-neck flask, add 6g of triethylamine, add the above solution dropwise at 100°C under mechanical stirring, and continue the reaction for 3 hours after the dropping is complete. The reaction was stopped, quenched by adding water, the organic layer was separated, and the aqueous layer was extracted with toluene. The organic layers were combined and desolventized to obtain 44.9 g of a white solid with a yield of 98.5% and a purity of 98.6% by HPLC.
Embodiment 2
[0030] Add 30g of pivalic acid into a 250mL three-necked flask, add 30mL of toluene to dissolve, raise the temperature to 80°C, and add 43g of solid phosgene in batches. After the addition was complete, the reaction was continued for 2 hours, and the released HCl gas was passed into water. Cool down to room temperature and set aside.
[0031] Add 28g of methylthiosemicarbazide and 30mL of toluene into a 500mL four-neck flask, add 81g of triethylamine, add the above solution dropwise at 100°C under mechanical stirring, and continue the reaction for 3 hours after the drop is complete. The reaction was stopped, quenched by adding water, the organic layer was separated, and the aqueous layer was extracted with toluene. The organic layers were combined and precipitated to obtain 44.3 g of a white solid with a yield of 97.1% and a purity of 97.6% by HPLC.
Embodiment 3
[0033] Add 30g of pivalic acid into a 250mL three-necked flask, add 30mL of toluene to dissolve, raise the temperature to 80°C, and add 45g of dimeric phosgene in batches. After the addition was complete, the reaction was continued for 2 hours, and the released HCl gas was passed into water. Cool down to room temperature and set aside.
[0034] Add 28g of methylthiosemicarbazide and 30mL of toluene into a 500mL four-neck flask, add 4g of triethylamine, add the above solution dropwise at 100°C under mechanical stirring, and continue the reaction for 3 hours after the drop is complete. The reaction was stopped, quenched by adding water, the organic layer was separated, and the aqueous layer was extracted with toluene. The organic layers were combined and desolventized to obtain 45.1 g of a white solid with a yield of 98.9% and a purity of 98.8% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com