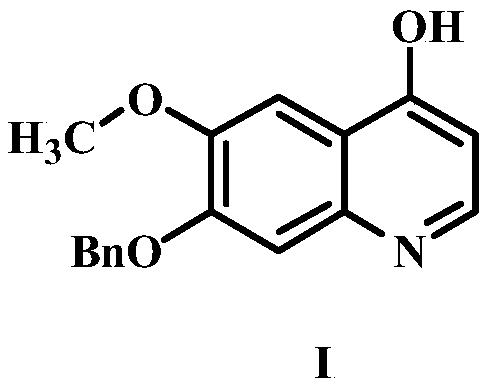
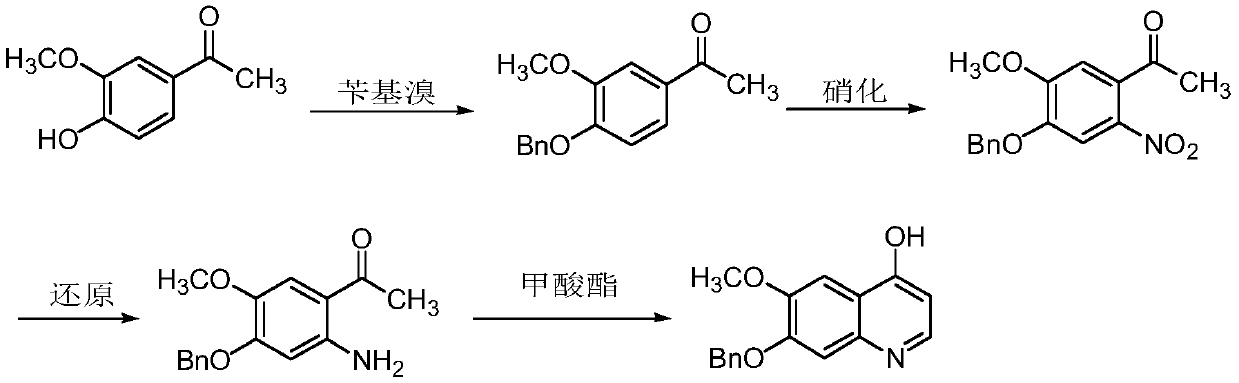
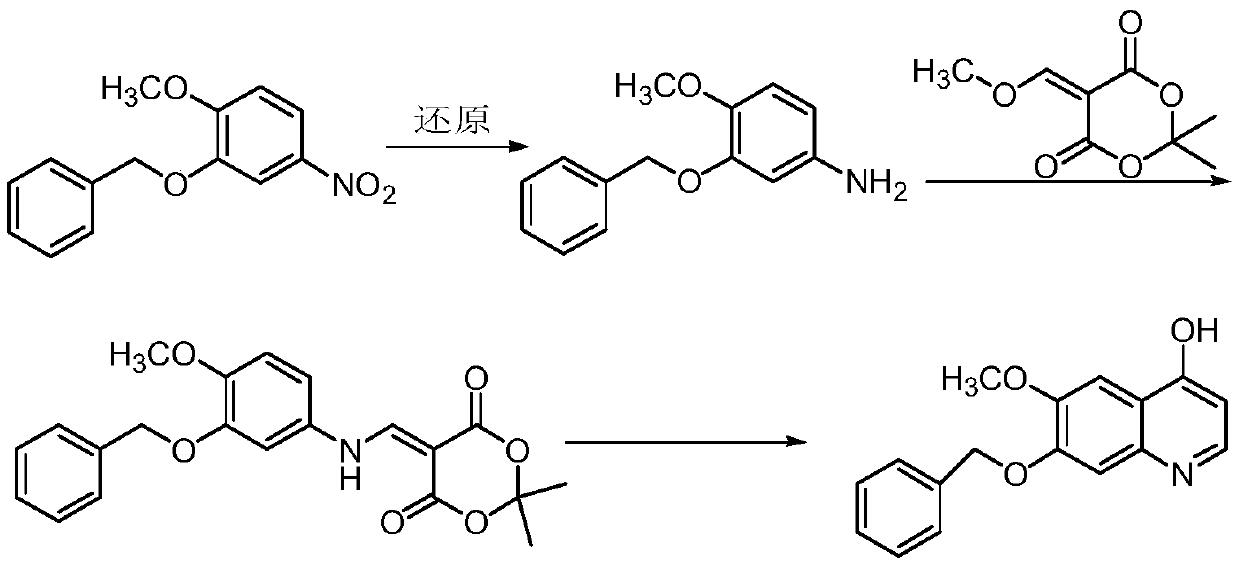
The preparation method of 7-benzyloxy-6-methoxy-4-hydroxyquinoline
A technology of hydroxyquinoline and methoxyphenyl, which is applied in the field of organic compound synthesis, can solve the problems of long reaction time, high energy consumption, and high requirements for production equipment, and achieves the effects of simple operation, high yield and no special requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of 3-benzyloxy-4-methoxynitrobenzene
[0052] Add 2-methoxyl-5-nitrophenol (10.0g, 59.2mmol), anhydrous potassium carbonate (12.2g, 88.4mmol) and N,N-dimethylformamide 100ml successively in a 250ml three-necked flask, Benzyl bromide (11.1g, 64.9mmol) was added dropwise under stirring, and the temperature was raised to 40°C for 4 hours after the drop, during which the reaction was detected by thin layer chromatography (ethyl acetate:petroleum ether (V:V)=2:1) completely. After the reaction was completed, after cooling to room temperature, the reaction solution was poured into 500 ml of ice water, stirred, filtered with suction, and dried to obtain 14.1 g of a light yellow solid with a yield of 93.1%.
[0053] 1 H-NMR(400MHz,DMSO-d6)δ:3.88(s,3H,-CH 3 ); 5.18(s,2H,-CH 2 ); 7.13~7.15(d,1H,ArH); 7.33~7.46(m,5H,ArH); 7,81~7,82(d,1H,ArH); 7.87~7.90(dd,1H,ArH)) .
[0054] Preparation of 3-benzyloxy-4-methoxyaniline
[0055] Add 3-benzyloxy-4-methoxynitrobenz...
Embodiment 2
[0062] The preparation of 3-benzyloxy-4-methoxynitrobenzene and 3-benzyloxy-4-methoxyaniline is according to Example 1.
[0063] Preparation of 7-benzyloxy-6-methoxy-4-hydroxyquinoline
[0064] First add 3,3-diethoxy ethyl propionate (4.84ml, 25.0mmol) and 0.57g of anhydrous p-toluenesulfonic acid into a 100ml three-necked flask, stir at room temperature for 15 minutes, then add 3-benzyl Oxy-4-methoxyaniline (5.73g, 25.0mmol) was heated to 60°C for 5 hours, during which the reaction was detected by thin layer chromatography (ethyl acetate:petroleum ether (V:V)=1:2) . Cool to room temperature to obtain ethyl 3-(3-benzyloxy-4-methoxyphenyl)iminopropionate, which can be directly subjected to ring closure without separation and purification.
[0065] Add 49ml of diphenyl ether to the above reaction product, slowly heat to 180°C for a ring closure reaction for 6 hours, thin layer chromatography (ethyl acetate:petroleum ether (V:V)=2:1) detects that the reaction is complete, c...
Embodiment 3
[0067] The preparation of 3-benzyloxy-4-methoxynitrobenzene and 3-benzyloxy-4-methoxyaniline is according to Example 1.
[0068] Preparation of 7-benzyloxy-6-methoxy-4-hydroxyquinoline
[0069] First add 3,3-diethoxy ethyl propionate (4.84ml, 25.0mmol) and 0.1g of anhydrous p-toluenesulfonic acid into a 100ml three-necked flask, stir at room temperature for 15 minutes, then add 3-benzyl Oxy-4-methoxyaniline (5.73g, 25.0mmol) was heated to 95°C for 5 hours, during which the reaction was detected by thin layer chromatography (ethyl acetate:petroleum ether (V:V)=1:2) . Cool to room temperature to obtain ethyl 3-(3-benzyloxy-4-methoxyphenyl)iminopropionate, which can be directly subjected to ring closure without separation and purification.
[0070] Add 25ml of diphenyl ether to the above reaction product, slowly heat to 180°C for a ring closure reaction for 6 hours, thin layer chromatography (ethyl acetate:petroleum ether (V:V)=2:1) detects that the reaction is complete, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com