Method for synthesizing 1-alkyl-2-alkoxyl-3-indole aldoxime derivant
A technology of indole aldoxime and alkoxy is applied in the field of synthesizing new compounds of 1-alkyl-2-methoxy-3-indole aldoxime, which can solve the problems of slow reaction speed, long production cycle and the like, Achieve the effect of low cost, low environmental burden and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
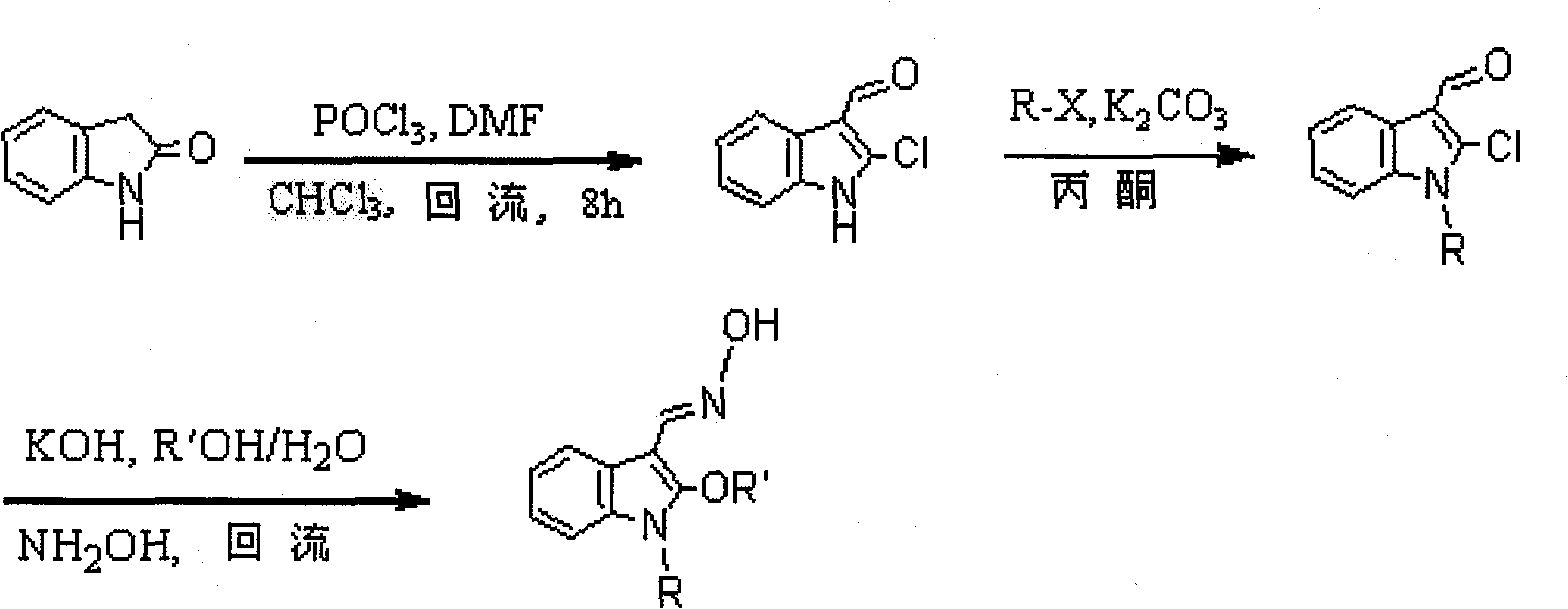
[0031] Take indolinone as substrate to obtain 1-alkyl-2-chloro-3-indole aldehyde through Vilsmeier-Hacck formylation reaction and alkylation reaction, in the presence of potassium hydroxide and fatty alcohol, the described Fatty alcohol can be reacted with methanol or ethanol or propanol or butanol or pentanol or cyclohexanol at 50-80°C, and the reaction temperature should be kept at 70°C or under reflux; the reaction time is 1-3 hours. Layer chromatography TLC monitoring, after the reaction is complete, directly add hydroxylamine hydrochloride to the mixture without any treatment (that is, the intermediate product obtained in the alkoxylation reaction can directly add hydroxylamine hydrochloride to carry out the next reaction) and the oximation reaction can occur. The temperature is constant, the reaction time is 1-2 hours, and the ratio of the 1-alkyl-2-chloro-3-indolaldehyde, hydroxylamine hydrochloride, potassium hydroxide, fatty alcohol and water is 1mmol: 2mmol: 2mmol: 5-...
Embodiment 2
[0035] POCl 3 (17.5 mL) was added drop by drop to dimethylformamide DMF (17.5 mL) under vigorous stirring in an ice bath. Then 2-indolinone (10g, 75.2mol) was dissolved in 100mL CHCl 3 , then slowly dropwise added to POCl 3 and DMF mixture, vigorously stirred for half an hour. Stirring was then stopped and left overnight. The next day, after 8 hours of reflux under vigorous stirring, the CHCl was distilled off with a rotary evaporator. 3 , and then add 200 mL of water to it. After standing overnight, the precipitate was filtered and recrystallized with ethanol to obtain 5.5 g of orange-red needle-like crystals, namely 2-chloro-3-indolaldehyde, with a yield of 40.8%.
[0036] With the above product and K 2 CO 3 Dissolve in an appropriate amount of acetone at a molar ratio of 1:1.5 (add a small amount of potassium iodide to the reaction solution when the alkylating agent is ethyl bromide and benzyl chloride), and then put it into a round-bottomed flask equipped with a sti...
Embodiment 3
[0039] With 1-ethyl-2-chloro-3-indole aldehyde as raw material reaction, other reaction conditions and methods are the same as in Example 1, to obtain 1-ethyl-2-methoxyl group-3-indole aldoxime, yield It is 67.7%, and its melting point is 102.0-103.0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com