Method for preparing Salen Ni
A technology of salicylaldehyde and Schiff base, which is applied in the preparation of Salen Ni and the field of one-pot preparation of chiral catalysts, which can solve the problem of increasing "three wastes" and raw material consumption, increasing operation steps and solvent consumption, and "three wastes" treatment capacity Major problems, to achieve the effect of simplifying the post-processing equipment, reducing the generation of by-products and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
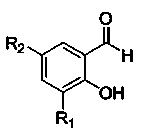
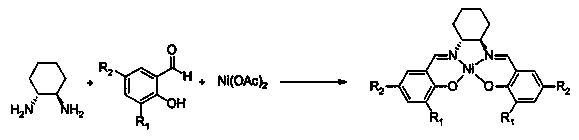
[0027] Add 22.8 g of chiral cyclohexanediamine and 100 mL of dichloromethane into a 1000 mL single-necked bottle, and add 93.6 g of 3,5-di-tert-butyl salicylaldehyde dichloromethane dropwise at -20°C in 200 mL , 1.5 hours to drop, after the end of the drop, keep warm for 2 hours, add 49.6 grams of nickel acetate tetrahydrate aqueous solution (2.0 mol / L) 100mL, react at room temperature for 12 hours, separate the reaction mixture after the reaction, and wash with 30 mL of dichloromethane The aqueous phase was combined with the organic phase, and the solvent was distilled off to obtain a crude product. The crude product was recrystallized from pure ethanol to obtain a reddish-brown Salen Ni solid powder with a yield of 86%. The purity of the sample was greater than 99% as measured by specific rotation.
Embodiment 2
[0029] Add 22.8 g of chiral cyclohexanediamine (RR type) and 100 mL of dichloromethane into a 1000 mL single-necked bottle, and add 70.2 g of 3,5-di-tert-butyl salicylaldehyde dichloride dropwise at -20 °C 200 mL of methane solution, drop it in 1.5 hours, keep it warm for 2 hours after the addition, add 49.6 grams of nickel acetate tetrahydrate aqueous solution (2.0 mol / L) 100 mL, react at room temperature for 12 hours, separate the reaction mixture after the reaction, 30 mL The aqueous phase was washed with dichloromethane, the organic phase was combined, and the solvent was distilled off to obtain a crude product. The crude product was recrystallized from pure ethanol to obtain a reddish-brown Salen Ni solid powder with a yield of 87%. The purity of the sample was greater than 99% as determined by specific rotation.
Embodiment 3
[0031]Add 22.8 g of chiral cyclohexanediamine (R R type) and 100 mL of dichloromethane into a 1000 mL single-necked bottle, and add 93.6 g of 3,5-di-tert-butyl salicylaldehyde dichloride dropwise at -20 °C 200 mL of methane solution, drop it in 1.5 hours, keep it warm for 2 hours after the addition, add 100 mL of 24.8 g nickel acetate tetrahydrate aqueous solution (1.0 mol / L), react at room temperature for 12 hours, separate the reaction mixture after the reaction, 30 mL The aqueous phase was washed with dichloromethane, the organic phase was combined, and the solvent was distilled off to obtain a crude product. The crude product was recrystallized from pure ethanol to obtain a reddish-brown Salen Ni solid powder with a yield of 41%. The purity of the sample was greater than 99% as determined by specific rotation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com