Application of 5,6,7,4'-tetrahydroxy flavone and derivatives thereof in preparation of drugs for preventing and treating hyperuricemia and gout
A technology for hyperuricemia and gout, applied in the field of medicine, can solve the problems of low activity of hyperuricemia and lower blood uric acid level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 : Effects of 5,6,7,4'-tetrahydroxyflavone and its derivatives on hyperuricemia mice
[0039] Healthy male Kunming mice, weighing 18-22 g, were provided by the Experimental Animal Center of Kunming Medical College (Experimental Animal Production License Number: SCXK (Dian) 2005-0008). Breeding conditions: room temperature is 22±2°C, relative humidity is 45-55%.
[0040]
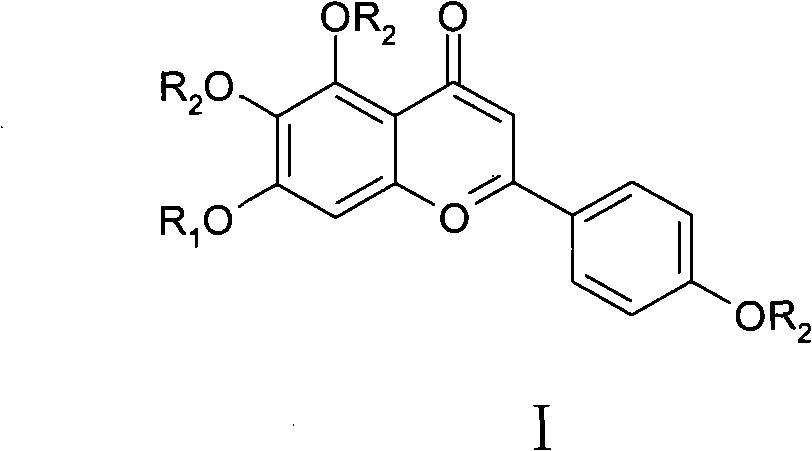
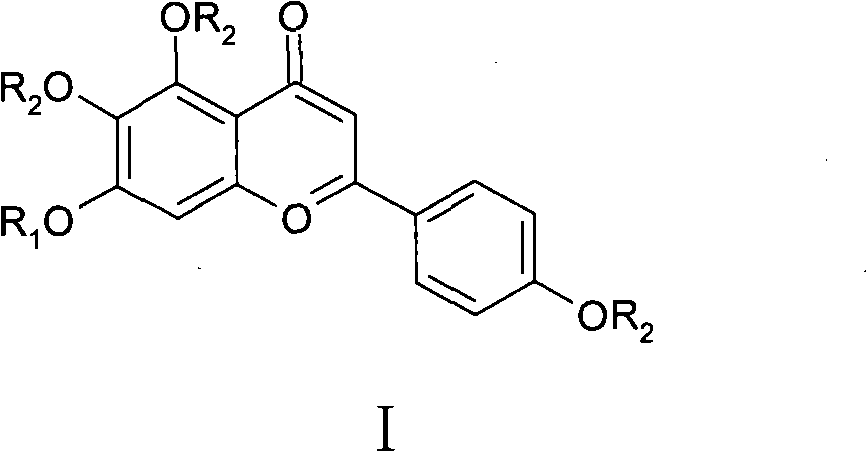
[0041] In formula Ⅰ, R 1 -H, -CH 3 、-COCH 3 or glucuronic acid; R 2 -H, -CH 3 or -COCH 3 .
[0042] Among them, when R 1 = Gluconic acid, R 2 =H, the corresponding compound is scutellarin, the number is DZHS-001;
[0043] When R 1 =R 2 =H, the corresponding compound is 5,6,7,4'-tetrahydroxyflavone, the number is DZHS-002;
[0044] When R 1 =R 2 =-CH 3 , the corresponding compound is 5,6,7,4'-tetramethoxyflavone, number DZHS-004;
[0045] When R 1 =R 2 =-COCH 3 , the corresponding compound is 4',5,6,7-tetraacetoxyflavone, number DZHS-006;
[0046]When R 1 = H, R 2 =-CO...
Embodiment 2
[0056] Example 2 : Study on the dose-effect relationship of 5,6,7,4'-tetrahydroxyflavone and its derivatives in reducing hyperuricemia
[0057] In order to evaluate the dose-effect relationship of 5,6,7,4'-tetrahydroxyflavone and its derivatives in reducing hyperuricemia, 5,6,7,4'-tetrahydroxyflavone (DZHS-002) and Dengzhan Anthocyanin (DZHS-001) conducts dose-effect relationship research, the specific method is as follows:
[0058] 110 healthy male Kunming mice, weighing 18-22 g, were provided by the Experimental Animal Center of Kunming Medical College (the experimental animal production license number: SCXK (Dian) 2005-2008). Animals were randomly divided into normal control group, hyperuricemia model group, 5,6,7,4'-tetrahydroxyflavone 2.5, 5, and 10 dose groups, scutellarin 2.5, 5, and 10 dose groups, and allopurinol 2.38mg / kg 3.13mg / kg Positive control group, 10 rats in each group. The test compound was formulated into a suspension with 0.5% sodium carboxymethylcellu...
Embodiment 3
[0065] Example 3 : Effect of 5,6,7,4'-tetrahydroxyflavone and its derivatives on xanthine oxidase in vitro
[0066] In order to evaluate the effect of 5,6,7,4'-tetrahydroxyflavone and its derivatives on xanthine oxidase, 5,6,7,4'-tetrahydroxyflavone (DZHS-002) and breviscapine B Xanthine oxidase (DZHS-001) was studied in vitro, the specific method is as follows:
[0067] Xanthine oxidase (EC 1.1.3.22) is a product of Sigma Company, prepared with PBS to an appropriate concentration for use. 5,6,7,4'-Tetrahydroxyflavone and its derivatives were prepared into a series of concentrations with PBS for future use, and DMSO was used to aid in dissolution. Xanthine oxidase assay kit is a product of Nanjing Jiancheng Bioengineering Institute.
[0068] After incubating 5, 6, 7, 4'-tetrahydroxyflavone and its derivative solutions with serial concentrations and xanthine oxidase at 37°C for 30 minutes, the activity of xanthine oxidase was measured with a xanthine oxidase assay kit, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com