Method for extracting lithium by processing lepidolite through alkali dissolution process
A technology of lepidolite and lepidolite powder, applied in the direction of improving process efficiency, etc., can solve the problems of large pollution, large energy consumption, and loss of money, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
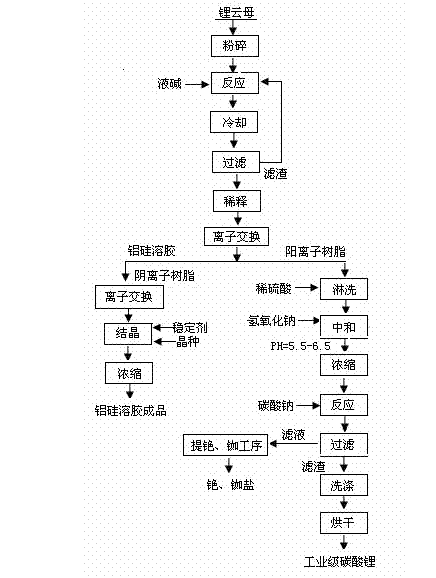
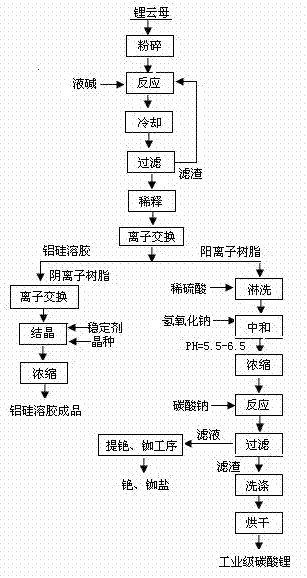
[0020] (1) Put lepidolite powder crushed to 700 meshes and 50% (wt%) strong alkali solution into the pressure-resistant reactor at a weight ratio of lepidolite powder: strong alkali solution = 1: 8, start stirring, and React at 250°C and 10 atmospheres for 8 hours, cool and filter. The filtered solids are returned to the reactor, and the reaction is continued next time.
[0021] (2) The reaction solution is diluted with water to a concentration of 2% (wt%), and the diluted material is ion-exchanged with a weakly acidic cation exchange resin to obtain cations in the system. The remaining alumina-silica sol is passed through a weakly basic anion exchange resin to remove fluoride ions in the solution, and then a small amount of sodium hydroxide is added as a stabilizer to adjust the pH to 8. After crystallization and concentration, the finished alumina-silica sol is sold directly.
[0022] (3) The ion-exchange resin that has adsorbed cations is first washed with water, then rins...
Embodiment 2
[0027] (1) Put lepidolite powder crushed to 100 meshes and 25% (wt%) strong alkali solution into the pressure-resistant reactor at a weight ratio of lepidolite powder: strong alkali solution = 1: 3, start stirring, and React at 100°C and 6 atmospheres for 2 hours, cool and filter. The filtered solids are returned to the reactor, and the reaction is continued next time.
[0028] (2) The reaction solution is diluted with water to a concentration of 5% (wt%), and the diluted material is ion-exchanged with a weakly acidic cation exchange resin to obtain cations in the system. The remaining alumina-silica sol is passed through a weakly basic anion exchange resin to remove fluoride ions in the solution, and then a small amount of sodium hydroxide is added as a stabilizer to adjust the pH to 8. After crystallization and concentration, the finished alumina-silica sol is sold directly.
[0029] (3) The ion-exchange resin that has adsorbed cations is first washed with water, then rinse...
Embodiment 3
[0034] (1) Put lepidolite powder crushed to 200 meshes and 35% (wt%) strong alkali solution into the pressure-resistant reactor according to the ratio of lepidolite powder: strong alkali solution = 1: 6 by weight, and start stirring. React at 190°C and 8 atmospheres for 6 hours, cool and filter. The filtered solids are returned to the reactor, and the reaction is continued next time.
[0035] (2) The reaction solution is diluted with water to a concentration of 10% (wt%), and the diluted material is ion-exchanged with a strongly acidic cation exchange resin to obtain cations in the system. The remaining alumina-silica sol is passed through a weakly basic anion exchange resin to remove fluoride ions in the solution, and then a small amount of sodium hydroxide is added as a stabilizer to adjust the pH to 8. After crystallization and concentration, the finished alumina-silica sol is sold directly.
[0036] (3) The ion exchange resin that has adsorbed cations is first washed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com