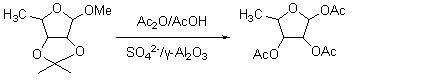
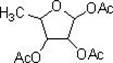
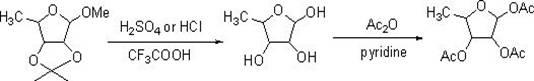
Method for synthesizing 1,2,3-O-triacetyl-5-deoxy-D-ribofuranose by using solid acid SO4<2->/gamma-Al2O3 as catalyst
A technology of ribofuranose and triacetyl, which is applied in the field of solid acid SO42-/γ-AL2O3 catalytic synthesis of 1, 2, 3-O-triacetyl-5-deoxy-D-ribofuranose, which can solve the complex post-processing , a large amount of waste water, environmental pollution and other problems, to achieve the effect of simple post-processing, no corrosion of equipment, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Dissolve 5.0 g, 26.6 mmol, of 1-methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranose in 50 ml of acetic anhydride / acetic acid at an equal volume ratio, and add 0.5 g of solid acid SO 4 2- / γ-Al 2 o 3 , stirred at room temperature for 1 h, raised the temperature to 50° C., continued to react for 2 h, then added 25 ml of water, filtered, and the filtrate was adjusted to pH 7 with saturated sodium bicarbonate. Then extract with 50ml of dichloromethane, separate the layers, wash the organic phase with 50ml of saturated brine, dry over anhydrous sodium sulfate, evaporate the solvent under conventional reduced pressure, and concentrate to dryness to obtain the target product 1,2,3-O-triacetyl 5.8 g of base-5-deoxy-D-ribofuranose, the yield was 85.6%, and the purity by gas chromatography was 95.1%.
Embodiment 2
[0015] By the method of embodiment 1, reaction temperature is changed into 80 ℃, other methods are unchanged. The target product 1, 2, 3-O-triacetyl-5-deoxy-D-ribofuranose was 6.1 g, the yield was 90.0%, and the purity by gas chromatography was 93.6%.
Embodiment 3
[0017] According to the method of Example 1, after stirring at room temperature for 2 hours, the reaction was carried out at 50° C. for 3 hours, and the other methods remained unchanged. The target product 1, 2, 3-O-triacetyl-5-deoxy-D-ribofuranose was 6.3 g, the yield was 93.0%, and the gas chromatography purity was 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com