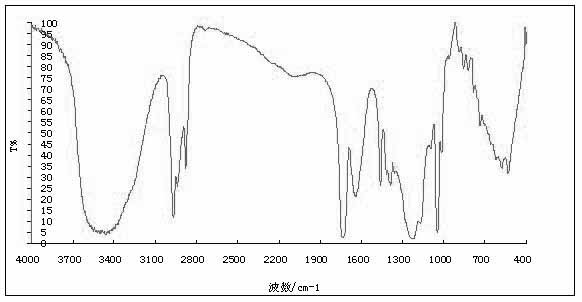
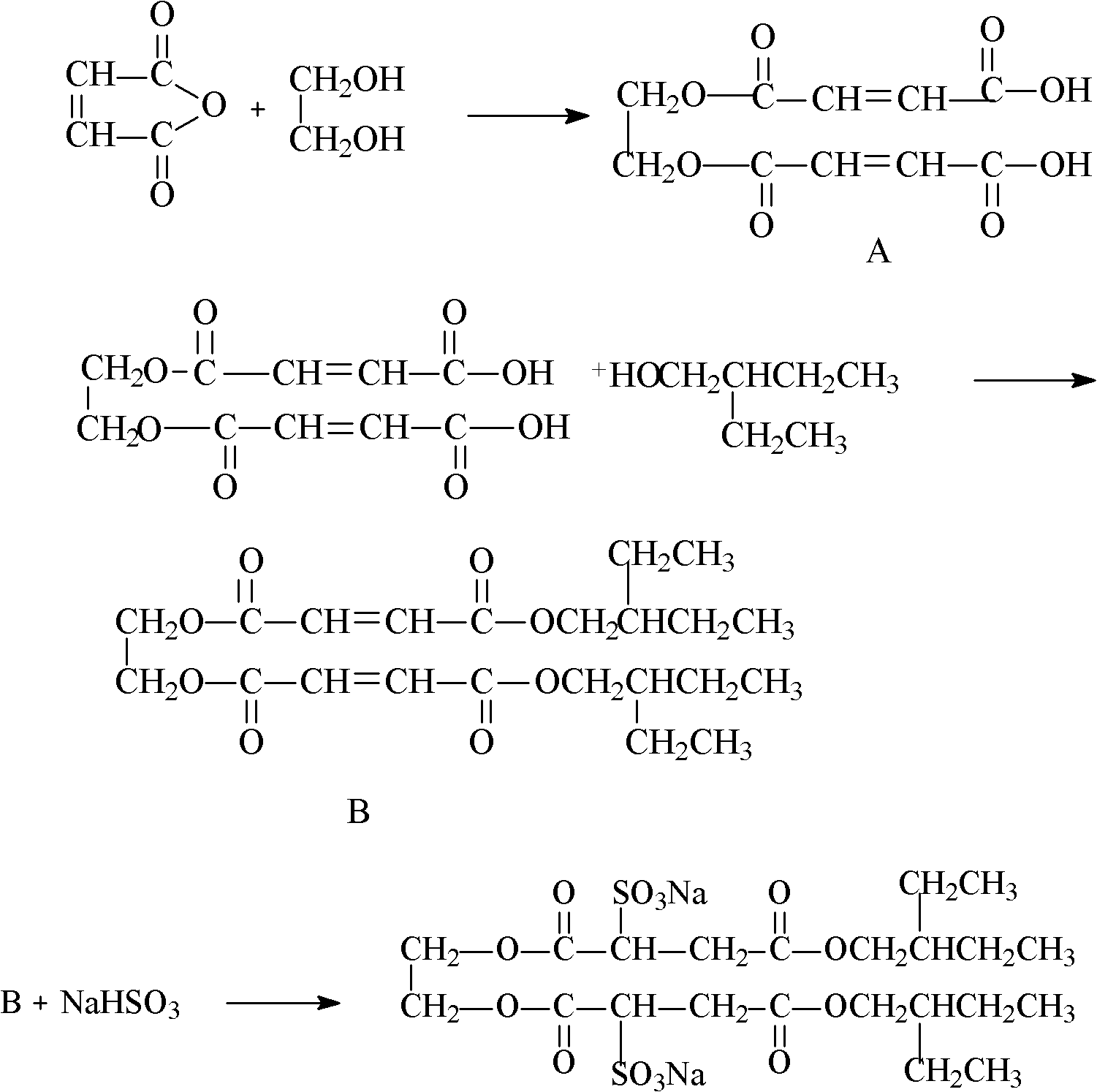Method for preparing disodium ethylene glycol bis(2-ethyl-1-butyl)sulfosuccinate
A technology of bissulfosuccinic acid and ethylene glycol, applied in the preparation of sulfonates, organic chemistry and other directions, can solve problems such as no literature reports, and achieve the requirements of short reaction time, reduced consumption, and reduced equipment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] A kind of preparation method of di(2-ethyl-1-butyl) sodium ethylene glycol bissulfosuccinate, comprises the following steps: the first step monoesterification reaction, by maleic anhydride and ethylene glycol molar ratio Take ethylene glycol and maleic anhydride in the ratio of 2.1: 1.0 and add them to the reaction kettle equipped with stirrer, thermometer and reflux condenser, then add the carbon-based solid acid catalyst (carbon-based solid acid catalyst) that accounts for 2% of the maleic anhydride mass. The preparation method of acid catalyst: add soluble starch and p-toluenesulfonic acid in beaker by m (p-toluenesulfonic acid): m (starch)=1: 2, heating dissolves with distilled water. Treat that solution is clear and transparent, cool to room temperature, use Rotary evaporator evaporated into a viscous colloid, placed in a muffle furnace and carbonized at 200 ° C for 8 h. Grinding, sieving through an 80-mesh sieve to obtain a carbon-based solid acid catalyst), nitrog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com