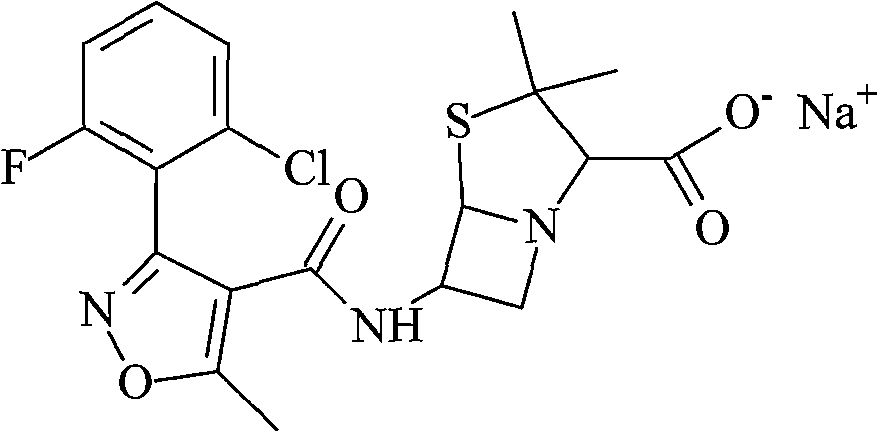
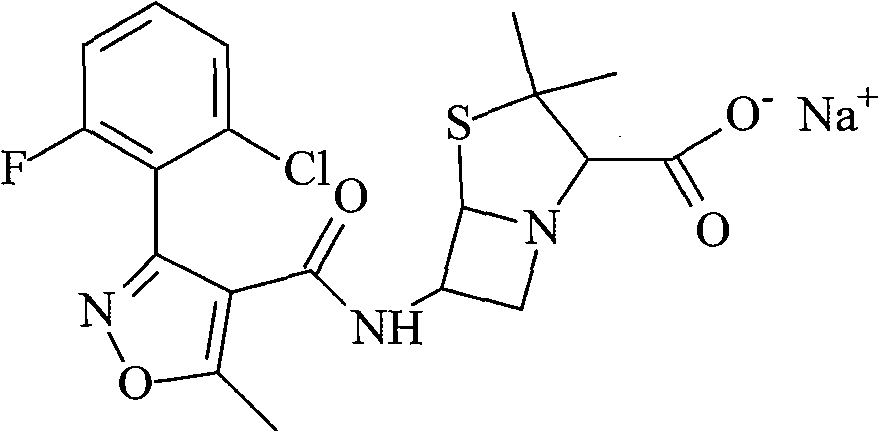
Flucloxacillin sodium compound and preparation method thereof
The technology of flucloxacillin sodium and compound is applied in the field of flucloxacillin sodium compound and preparation method thereof, which can solve the problems of low purity of flucloxacillin sodium, and achieve the effects of easy industrial production, high product purity, and improvement of clinical adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Get flucloxacillin sodium crude product 10g (content 89.8%) and dissolve in 100ml water, stir, make it dissolve completely, then add 25ml ether, stir, extract 2 times, liquid separation removes the ether phase that contains impurity, collects water phase.
[0039] Add 20 ml of 1 mol / l sodium methoxide to the water phase, stir at 60°C for 1.5 hours, a solid precipitates, let stand to lower the temperature to room temperature, filter, and collect the filtrate.
[0040] The filtrate was separated and purified with an ICN allumina N neutral alumina chromatographic column with a particle size of 18-32 μm and a pore size of 6 nm, wherein the mobile phase used in the chromatographic column was methanol-water (70:30), and the flow rate was 1.5 ml / min , the column temperature is room temperature, and the detection wavelength is 225nm. Collect the parts of cloxacillin sodium in the eluate, concentrate under reduced pressure at 75° C., and dry in vacuo to obtain 8.8 g of refined fl...
Embodiment 2
[0044] Take flucloxacillin sodium raw material drug (Guilin South Medicine Co., Ltd., batch number H20090054) 10g (content 94.6%), dissolve in 100ml water, stir, make it dissolve completely, then add 50ml ether, stir, extract 3 times, separate liquid The ether phase containing impurities was removed and the aqueous phase was collected.
[0045] Add 20 ml of 1 mol / l potassium methoxide to the water phase, stir at 80°C for 1.5 hours, a solid precipitates, let stand to lower the temperature to room temperature, filter, and collect the filtrate.
[0046] The filtrate was separated and purified with an ICN allumina N neutral alumina chromatographic column with a particle size of 18-32 μm and a pore size of 6 nm, wherein the mobile phase used in the chromatographic column was methanol-water (70:30), and the flow rate was 1.5 ml / min , the column temperature is room temperature, and the detection wavelength is 225nm. Collect the parts of cloxacillin sodium in the eluate, concentrate u...
Embodiment 3
[0051] Take flucloxacillin sodium raw material drug (Zhejiang Jinhua Kangenbei Biopharmaceutical Co., Ltd., batch number H20080051) 10g (content 95.4%), dissolve in 100ml water, stir, make it dissolve completely, then add 30ml ether, stir, extract 3 times , separated to remove the ether phase containing impurities, and collected the water phase.
[0052] Add 20 ml of 1 mol / l potassium methoxide to the water phase, stir at 70°C for 1.5 hours, a solid precipitates, let stand to lower the temperature to room temperature, filter, and collect the filtrate.
[0053] The filtrate is separated and purified with a special neutral alumina chromatographic column for Baker column chromatography with 50-200 μm and a pore size of 6 nm, wherein the mobile phase used in the chromatographic column is methanol-water (70: 30), and the flow rate is 1.5 ml / min. The temperature is room temperature, the detection wavelength is 225nm, and the part of cloxacillin sodium in the eluate is collected, con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com