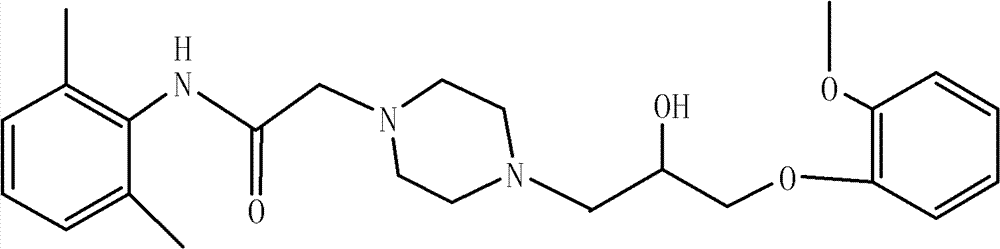
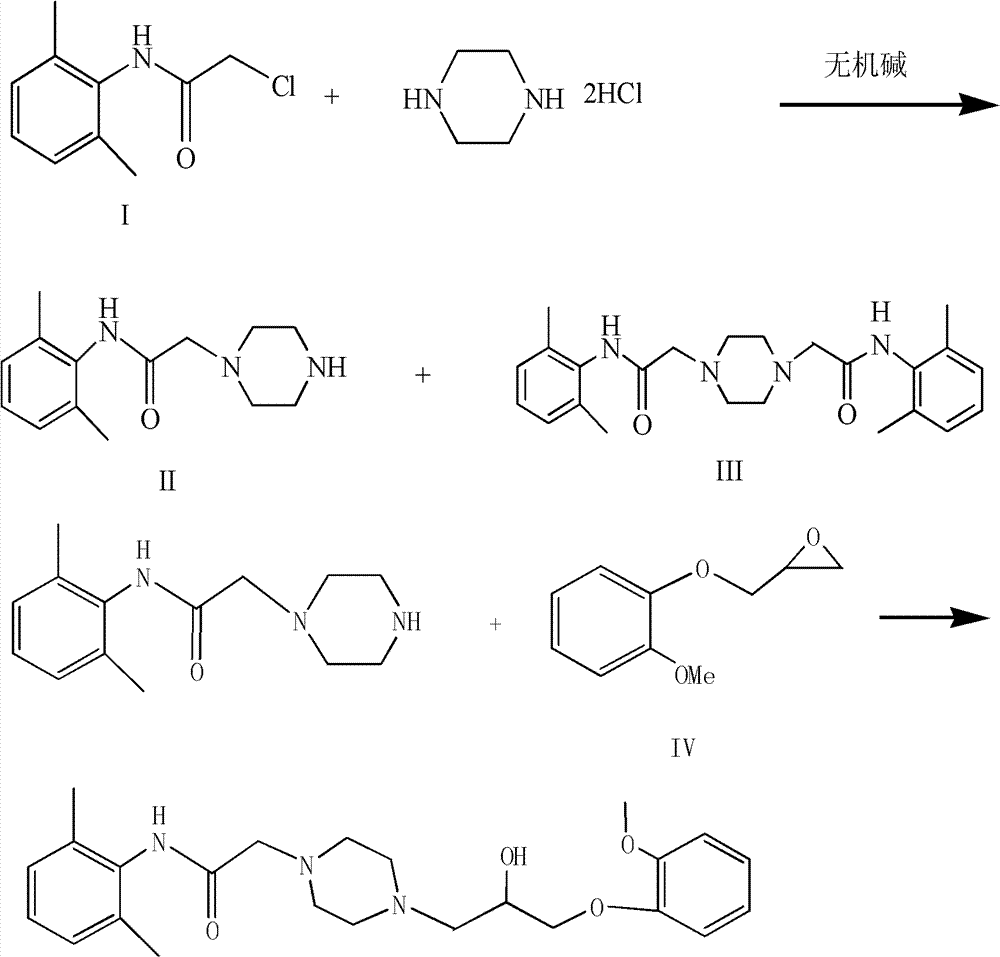
Preparation method of ranolazine
A technology of ranolazine and piperazine, applied in the field of preparation of ranolazine, can solve the problems of complicated processing, high risk, waste of piperazine and the like, and achieve the effects of simplifying process steps, reducing costs and improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 37g piperazine hydrochloride salt in the reaction bottle that 150ml 95% ethanol is housed, add dropwise 8mol / L sodium hydroxide solution 40ml while stirring, add 20g 2-chloro-N-(2,6- Xylyl)acetamide was heated to reflux, and after 1.5 hours of reaction, TLC monitored that the reaction was complete, and the heating was stopped. After cooling down to room temperature, add 35ml of concentrated hydrochloric acid dropwise to adjust the pH to 3-4, filter with suction, add 200ml of dichloromethane to the filtrate, add 60ml of 4mol / L sodium hydroxide solution dropwise while stirring, adjust the pH to 11-12, and separate the liquids , the aqueous layer was extracted twice with 100ml of dichloromethane respectively, the organic phases were combined, and the organic phase was washed twice with 80ml of water respectively, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 21.4g of off-white granular intermediates, Yield 85.6%.
[00...
Embodiment 2
[0024] Add 39g of piperazine hydrochloride salt into the reaction flask containing 150ml of 95% ethanol, add dropwise 55ml of 6mol / L sodium hydroxide solution while stirring, and add 20g of 2-chloro-N-(2,6- Xylyl)acetamide was heated to reflux, and after 1.5 hours of reaction, TLC monitored that the reaction was complete, and the heating was stopped. After cooling down to room temperature, add 35ml of concentrated hydrochloric acid dropwise to adjust the pH to 3-4, filter with suction, add 200ml of dichloromethane to the filtrate, add 60ml of 4mol / L sodium hydroxide solution dropwise while stirring, adjust the pH to 11-12, and separate the liquids , the aqueous layer was extracted twice with 100ml of dichloromethane, the organic phases were combined, and the organic phase was washed twice with 80ml of water, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 21.2 g of off-white granular intermediates. Yield 84.8%
[0025] After dis...
Embodiment 3
[0027] Add 35g of piperazine hydrochloride salt into the reaction flask containing 150ml of 95% ethanol, add 70ml of 4mol / L sodium hydroxide solution dropwise while stirring, and add 20g of 2-chloro-N-(2,6- Xylyl)acetamide was heated to reflux, and after 2 hours of reaction, TLC monitored that the reaction was complete, and the heating was stopped. After cooling down to room temperature, add 35ml of concentrated hydrochloric acid dropwise to adjust the pH to 3-4, filter with suction, add 200ml of dichloromethane to the filtrate, add 60ml of 4mol / L sodium hydroxide solution dropwise while stirring, adjust the pH to 11-12, and separate the liquids , the aqueous layer was extracted twice with 100ml dichloromethane respectively, the organic phases were combined, and the organic phase was washed twice with 80ml water respectively, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 21.0 g of off-white granular intermediates, Yield 84.1%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com