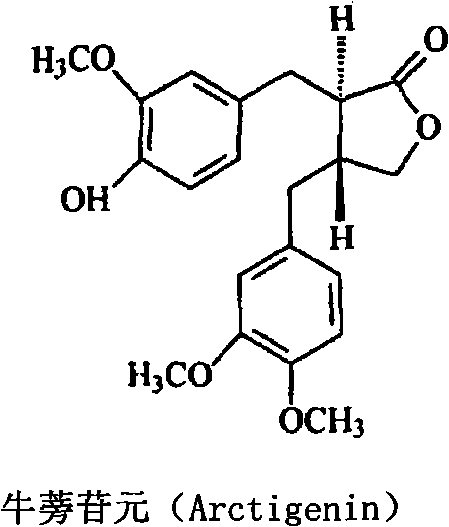
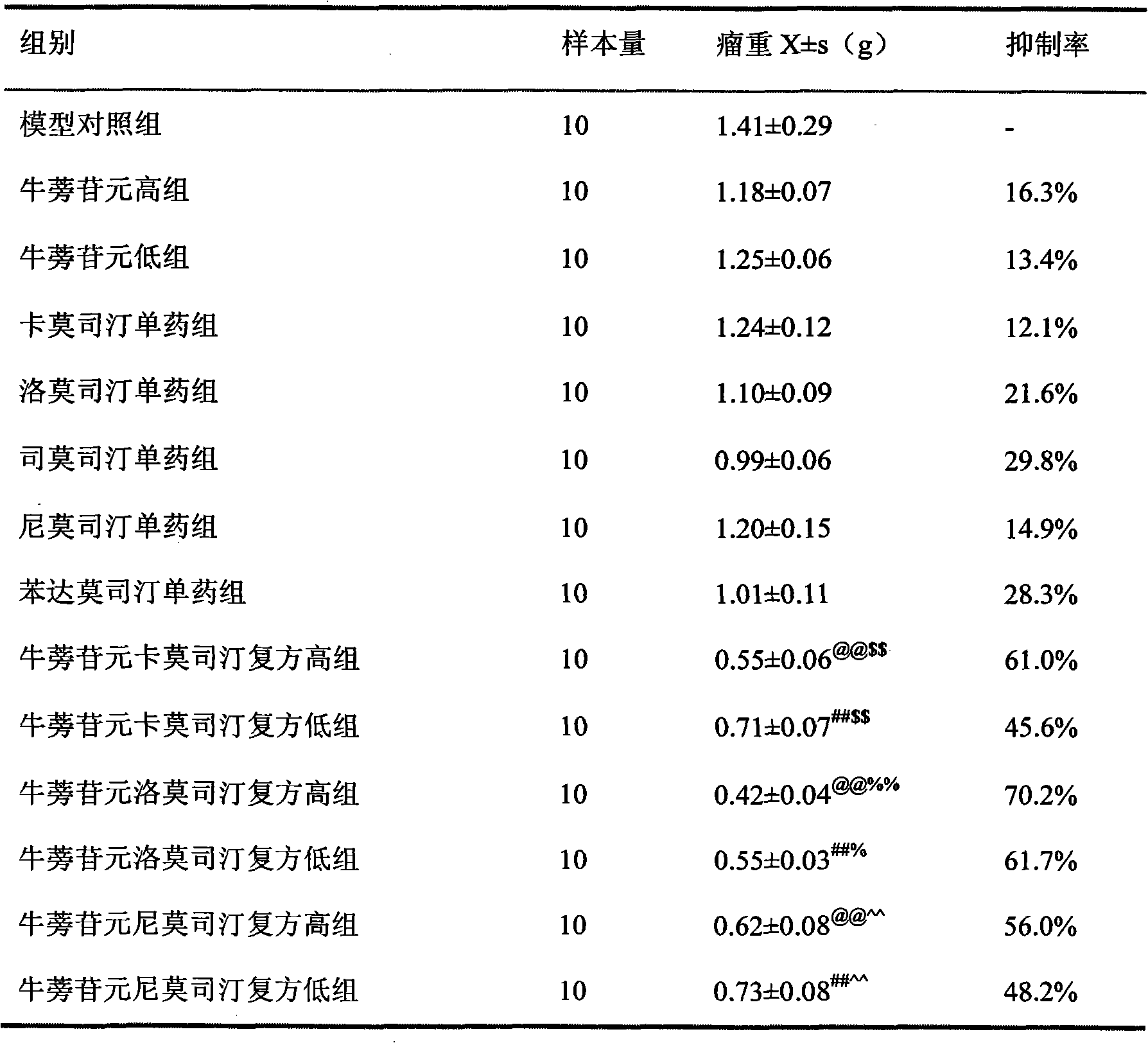
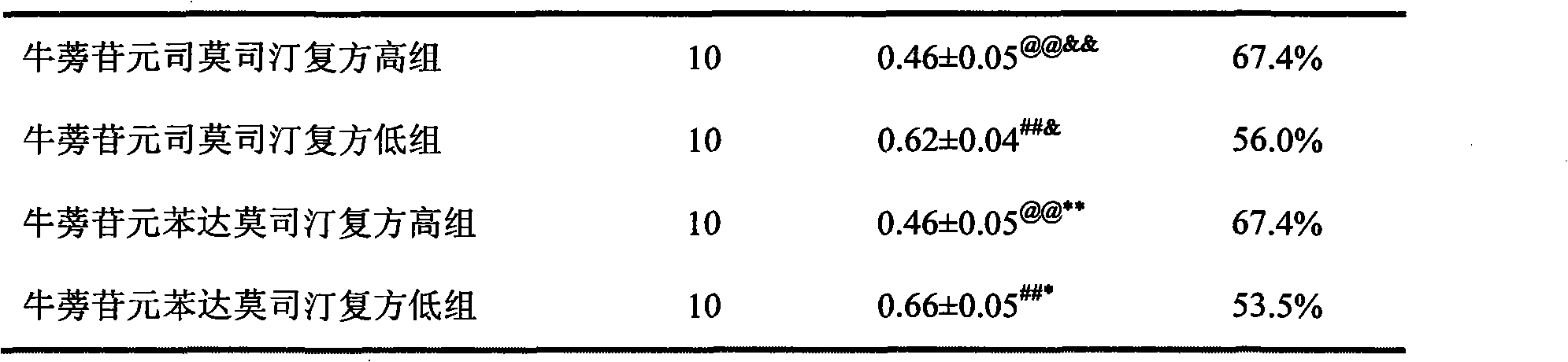
Anti-tumor medicine composition and purpose thereof
A technology of anti-tumor drugs and compositions, which is applied in the field of anti-tumor drug compositions and pharmaceutical compositions containing arctigenin and nitrosourea alkylating agents, which can solve the problems of large side effects, high treatment costs, and long duration of drug effects and other problems, to achieve the effect of reducing toxic and side effects, obvious synergistic effect, and clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 Preparation of arctigenin and carmustine composition microemulsion preparation
[0061] Table 2 Arctigenin and carmustine composition microemulsion preparation
[0062]
[0063] Preparation process: Weigh 30mg of caprylic acid glyceride and 50mg of emulsifier OP, add it to 20mg of propylene glycol, and mix well to obtain a suspension solution. Accurately weigh 25 mg arctigenin and 2.5 mg carmustine, add them to the suspension solution respectively, and ultrasonically dissolve arctigenin and carmustine to obtain a microemulsion of arctigenin and carmustine composition concentrate. The microemulsion concentrate obtained above is diluted with water according to the weight ratio of 1:10-20 to a clear solution to obtain the microemulsion.
Embodiment 2
[0064] Embodiment 2 Preparation of arctigenin and lomustine composition tablet
[0065] The tablet of table 3 arctigenin and lomustine composition
[0066]
[0067] Preparation process: pass arctigenin and lomustine through 80 mesh sieve respectively, weigh according to the prescription amount, mix evenly, then mix evenly with hydroxypropyl cyclodextrin in equal increments, pass through 60 mesh sieve, and then mix with Mix the rest of the excipients in the prescribed amount evenly, measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet.
Embodiment 3
[0068] Embodiment 3 Preparation of arctigenin and carmustine composition microemulsion preparation
[0069] Table 4 arctigenin and carmustine composition microemulsion preparation
[0070]
[0071]
[0072] Preparation process: Weigh the prescription amount of medium-chain fatty acid glycerides, polyoxyethylene castor oil EL-40, 1,2-propylene glycol, and absolute ethanol, mix and stir evenly, then add arctigenin and carmustine to dissolve, and also Ultrasonic treatment can be used to speed up the dissolution to obtain a clear concentrated solution, which is the arctigenin microemulsion concentrate. The microemulsion concentrate obtained above is diluted with water according to the weight ratio of 1:10-20 to a clear solution to obtain the microemulsion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com