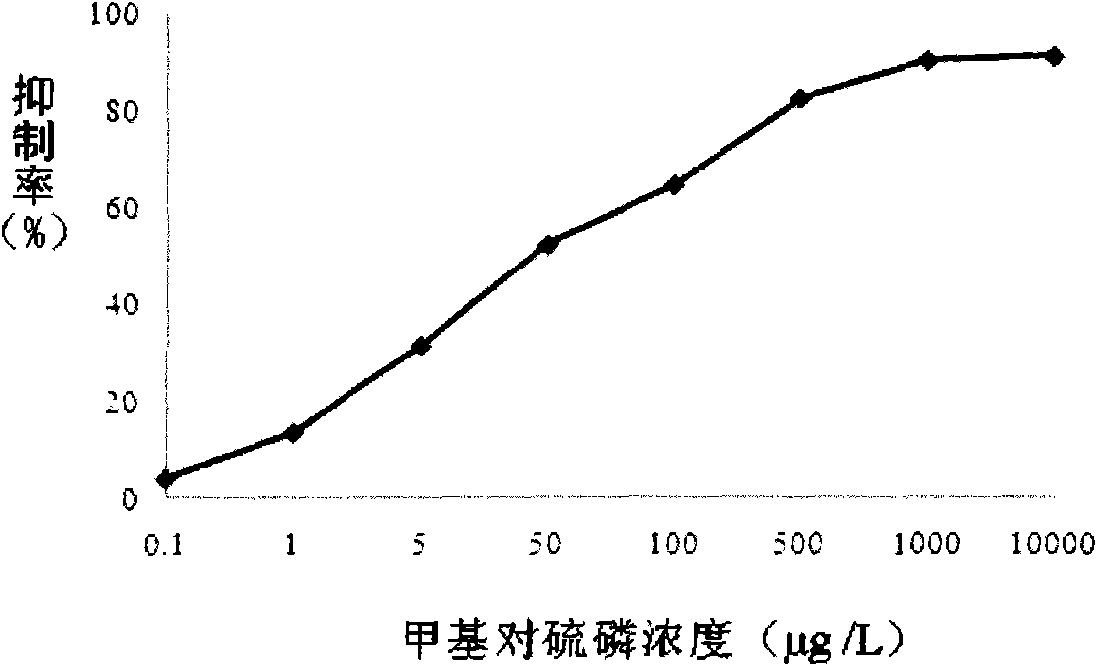
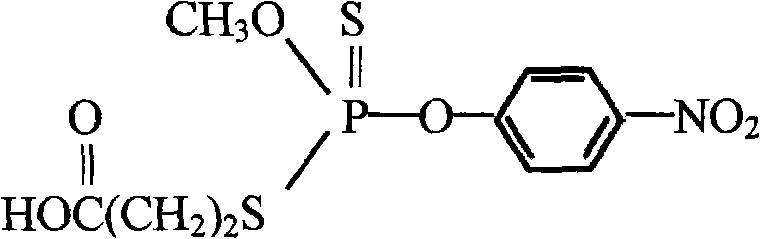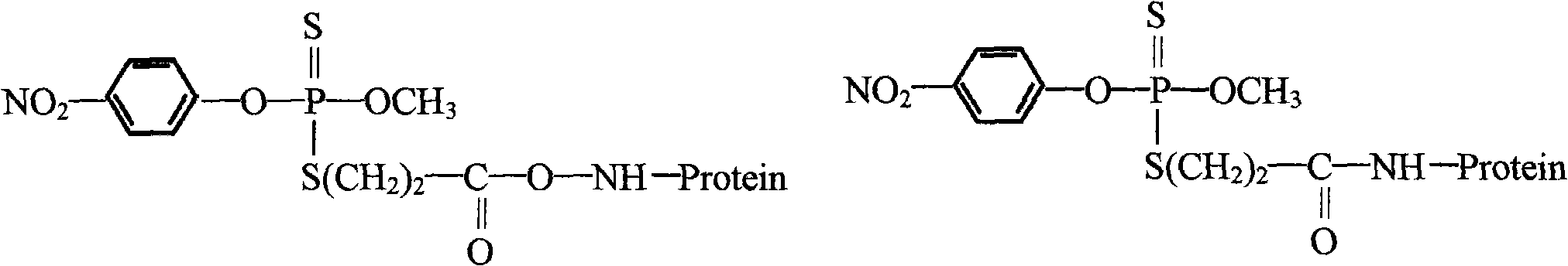Preparation method and application of parathion-methyl artificial hapten, artificial antigen and specific antibody
A methyl parathion, artificial hapten technology, applied in chemical instruments and methods, specific peptides, animal/human proteins, etc., can solve the problems of expensive equipment, high personnel and site requirements, and long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0009] Synthesis of hapten→preparation of artificial antigen→preparation of specific antibody→ELISA method establishment and
[0010] Identification → analysis of actual samples
[0011] 1. Synthesis of methyl parathion artificial hapten
[0012] Pesticide small molecules must be linked with macromolecules to stimulate animals to produce specific antibodies. Therefore, the synthesis and identification of haptens are the most basic and critical steps for the production of specific antibodies and the establishment of rapid detection technology for pesticide residues. On the one hand, the ideal hapten should have the characteristic structure of the analyte, especially the stereochemical characteristics. On the other hand, after the hapten is connected to the carrier, it should ensure that the characteristic structure of the analyte can be recognized and combined by the immune active cells to the greatest extent. To prepare antibodies with the expected selectivity. 1) Haptens ar...
Embodiment 1
[0033] Three weeks later, booster immunization was carried out, the immunization dose was 1.0 mg / kg, an equal volume of Freund's incomplete adjuvant was added, and multiple subcutaneous injections were performed on the back. After that, immunize once every three weeks with the same dose and method. From the third immunization, blood was collected from the rabbit's ear vein for one week each time, and the antibody titer and specificity were determined. Example 1: Synthesis of Hapten MP
[0034] Weigh 52.0g PSCl 3 , add 39.0g of anhydrous methanol dropwise at 15°C, react for 1h, and wash the reaction solution with water to obtain light yellow oil I→Weigh 40.0g of I, add 30.0g of p-nitrophenol, and add 3mol of tri-n-butyl under stirring Amine, after reacting for 20min, add 40mL of 6mol / L NaOH solution dropwise, and react for 1h. Filtered, extracted with ether, the ether phase was washed successively with NaOH aqueous solution with a mass fraction of 5 and distilled water, anhy...
Embodiment 2
[0037] Example 2: Synthesis of Hapten MP
[0038] Weigh 52.0g PSCl 3 , add 39.0g of anhydrous methanol dropwise at 15°C, react for 1h, and wash the reaction solution with water to obtain light yellow oil I→Weigh 40.0g of I, add 30.0g of p-nitrophenol, and add 3mol of tri-n-butyl under stirring Amine, after reacting for 20min, add 40mL of 6mol / L NaOH solution dropwise, and react for 1h. Filtered, extracted with ether, the ether phase was washed successively with NaOH aqueous solution with a mass fraction of 5 and distilled water, anhydrous MgSO 4 Drying, column chromatography, washing with petroleum ether / diethyl ether (7:1, volume ratio) to obtain light yellow oil II → weigh 6.2 g of 3-mercaptopropionic acid and 3.1 g of yellow oil II in 20 mL of dimethyl Add 2.0g of NaOH to the sulfoxide in a three-necked flask, and stir it magnetically to dissolve it; slowly heat up the oil bath to 100°C, keep it warm for 2 hours, then remove the oil bath, and after cooling to room tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com