Electrochemical deposition method for preparing super-hydrophobic and super-oleophilic surface
A super-hydrophobic and electrochemical technology, which is applied to the device for coating liquid on the surface, special surface, liquid chemical plating, etc., can solve the problems of unfavorable industrial production, complicated process, inconvenient operation, etc., and achieves good hydrophobic effect and film The effect of uniform thickness and not easy to fall off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Cleaning of copper or copper alloy sheets: Use absolute ethanol, acetone, 1.0M sodium hydroxide solution, and secondary water to ultrasonically clean the copper or copper alloy substrate, remove the oil on the surface, and dry it.
[0026] (2) Formation of micro-nano structure on the surface of copper or copper alloy sheet: immerse the cleaned copper or copper alloy substrate in 0.01M silver nitrate solution, and react at room temperature for 25 min. After the reaction, the substrate was washed with secondary water and dried with nitrogen.
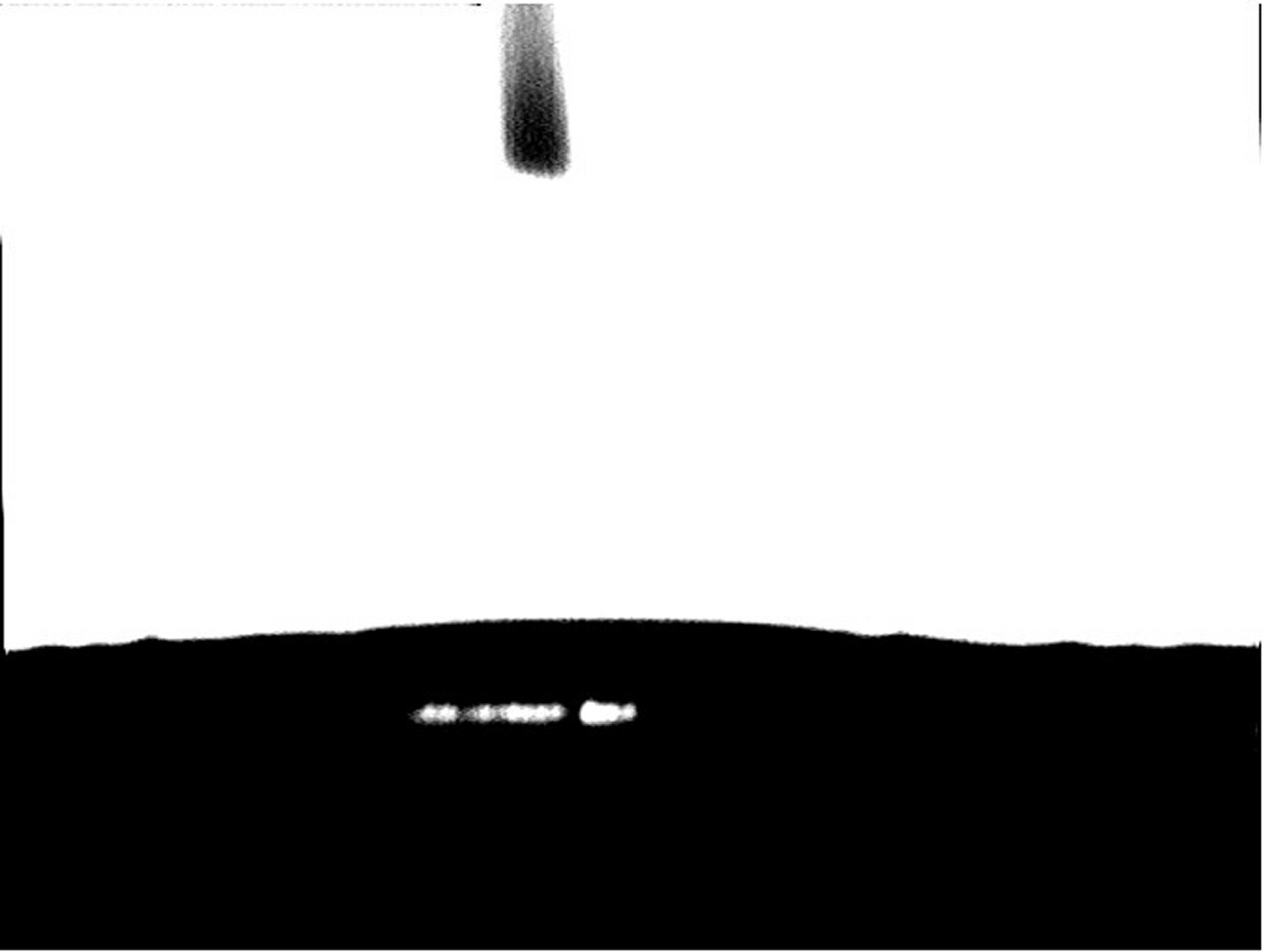
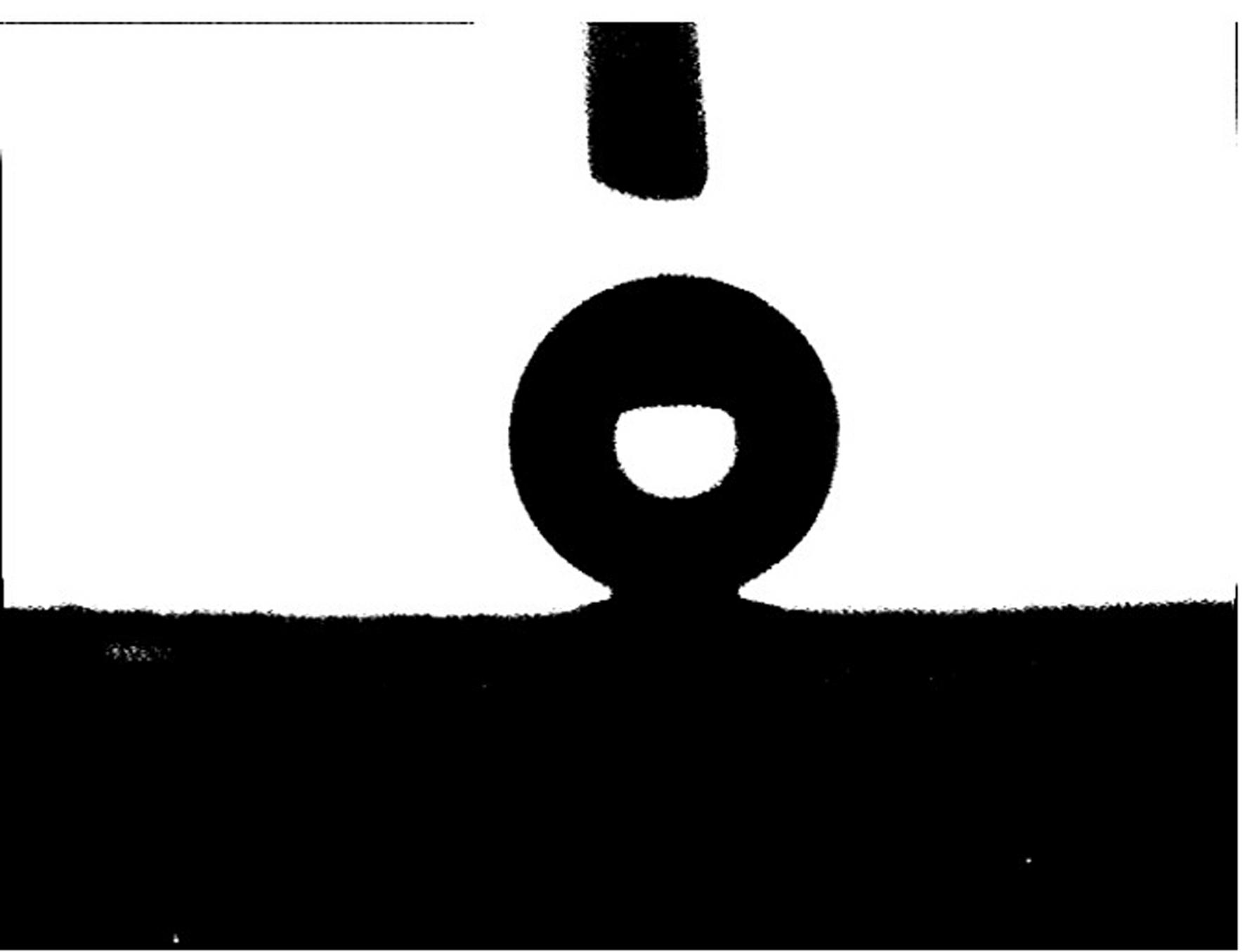
[0027] (3) Preparation of super-hydrophobic and super-oleophilic surface: immerse the above-mentioned treated copper or copper alloy substrate in an ethanol solution of dodecyl hydroxystearic acid with a mass concentration of 3%, and soak for 30 min at room temperature; after the reaction, The substrate was taken out, cleaned with absolute ethanol, and after drying, the surface water contact angle was measured to be 154°; the oi...
Embodiment 2
[0029] (1) Cleaning of copper or copper alloy substrate: same as in Example 1.
[0030] (2) Formation of micro-nanostructures on the surface of copper or copper alloy sheets: immerse the cleaned copper or copper alloy substrate in 0.01M silver nitrate solution, and react at room temperature for 30 min. After the reaction, the substrate was washed with secondary water and dried with nitrogen.
[0031] (3) Preparation of superhydrophobic and superoleophilic copper or copper alloy surface: immerse the above-mentioned treated copper or copper alloy substrate in an ethanol solution of dodecyl hydroxystearic acid with a mass concentration of 1%, and soak for 1 hour at room temperature; after the reaction , the substrate was taken out, cleaned with absolute ethanol, and after drying, the surface water contact angle was measured to be 156°; the oil droplets spread rapidly, and the oil contact angle was less than 5°.
Embodiment 3
[0033] (1) Cleaning of copper or copper alloy substrate: same as in Example 1.
[0034] (2) Formation of micro-nano structure on the surface of copper or copper alloy sheet: immerse the cleaned copper or copper alloy substrate in 0.01M silver nitrate solution, and react at room temperature for 25 min; after the reaction, the substrate is washed with secondary water , blow dry with nitrogen.
[0035] (3) Preparation of superhydrophobic and superoleophilic copper or copper alloy surface: immerse the above-mentioned treated copper or copper alloy substrate in an ethanol solution of dodecyl hydroxystearic acid with a mass concentration of 1%, and soak for 2 hours at room temperature; After that, the substrate was taken out, cleaned with absolute ethanol, and after drying, the surface water contact angle was measured to be 158°; the oil droplets spread rapidly, and the oil contact angle was close to 0°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com