Method for preparing isoquinoline ketone and derivatives thereof
A compound and reaction technology, which is applied in the field of preparation of isoquinolinone and its derivatives, can solve the problems of harsh reaction conditions, many by-products, difficult purification, etc., and achieve high synthesis yield, simple operation, and simple and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
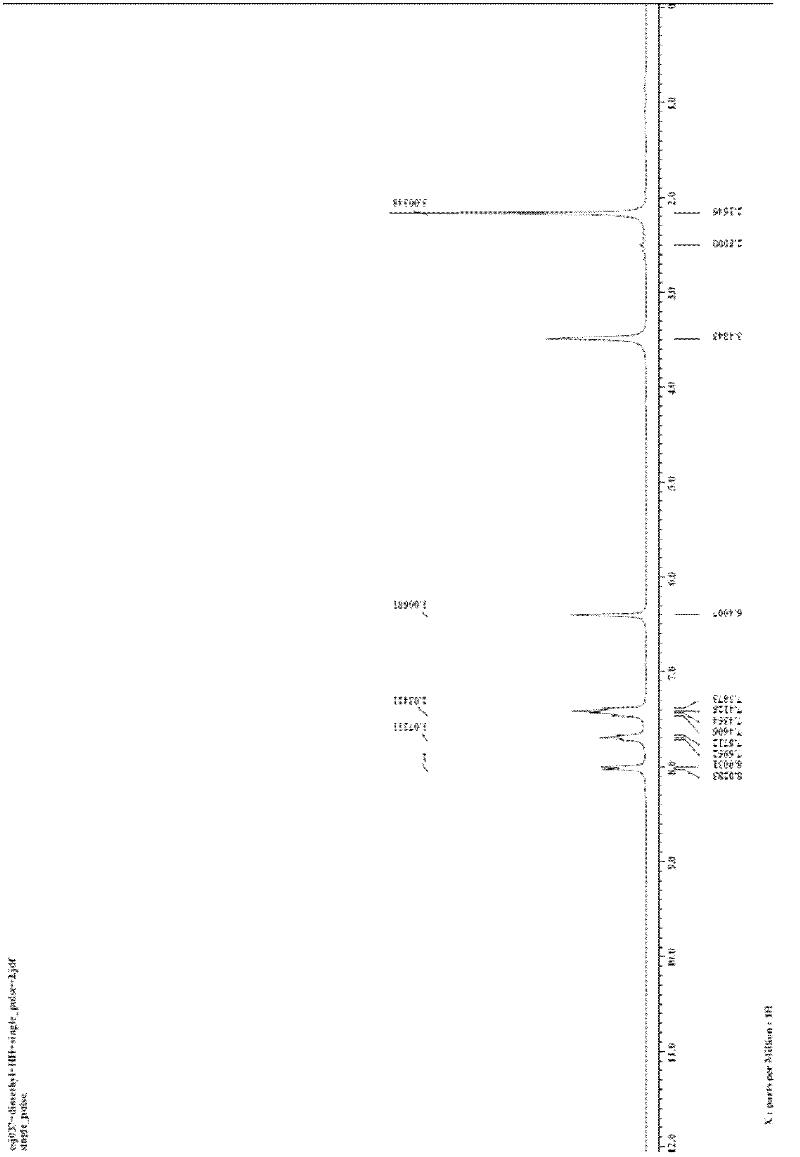
[0023] The preparation of embodiment 1,3-methylisoquinolinone (R in structural formula I 1 = methyl, R 2 =R=H)
[0024] In the 25mL reactor, add o-iodobenzamide (1.0mmol, 248.0mg), CuI (0.1mmol, 19.0mg), K 3 PO 4 (2.0mmol, 424.0mg), finally add acetylacetone (1.0mmol, 102.0μL), DMF (2mL), seal the reactor, and stir at 120°C for 24h. After the reaction system was cooled, 5 mL of water was added to quench the reaction, and 15 mL of ethyl acetate was used to extract three times. The filtrates were combined and washed twice with water and once with saturated brine. Magnesium sulfate was added to dry for 30 minutes, filtered, and the filtrate was concentrated by rotary evaporation to obtain a crude product. The crude product was separated by column (200-300 mesh silica gel) with a mixture of petroleum ether:ethyl acetate=5:1 (v / v) as eluent to obtain a yellow solid product 3-methylisocyanate with a purity greater than 99%. Quinolinone 102.0mg, isolated yield 65%.
[0025] The...
Embodiment 2
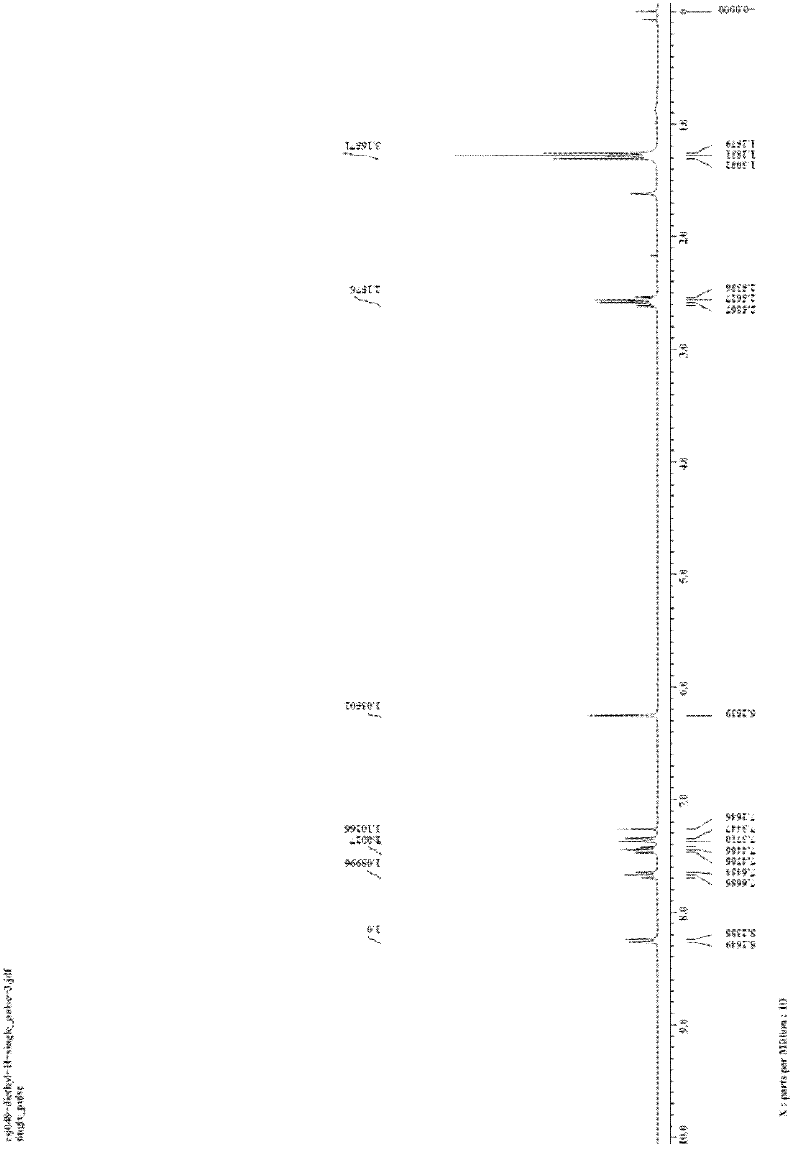
[0029] The preparation of embodiment 2,3-ethylisoquinolinone (R in structural formula I 1 = ethyl, R 2 =R=H)
[0030] In the reactor of 25mL, add o-iodobenzamide (1.0mmol, 248.0mg) successively, CuI (0.1mmol, 20.0mg), K 3 PO 4 (2.0mmol, 424.0mg), finally added 3,5-heptanedione (1.0mmol, 138.0μL), DMF (2mL), sealed the reactor, and stirred at 120°C for 24h. After the reaction system was cooled, 5 mL of water was added to quench the reaction, and 15 mL of ethyl acetate was used to extract three times. The filtrates were combined and washed twice with water and once with saturated brine. Magnesium sulfate was added to dry for 30 minutes, filtered, and the filtrate was concentrated by rotary evaporation to obtain a crude product. The crude product was separated by column (200-300 mesh silica gel) with a mixture of petroleum ether:ethyl acetate=10:1 (v / v) as eluent to obtain a yellow solid product 3-ethylisocyanate with a purity of more than 99%. Quinolinone 98.0 mg, isolated ...
Embodiment 3
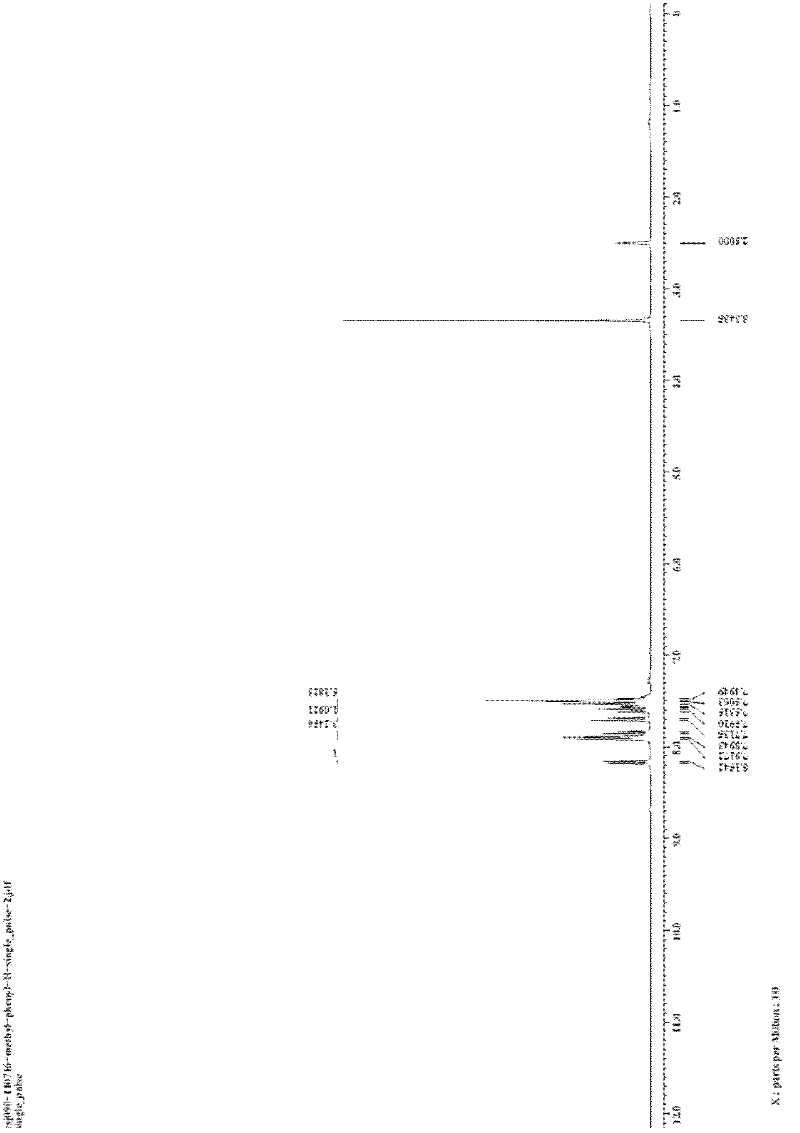
[0035] The preparation of embodiment 3,3-phenylisoquinolinone (R in structural formula I 1 = phenyl, R 2 =R=H)
[0036] In a 25mL reactor, add o-iodobenzamide (1.0mmol, 248.0mg), benzoylacetone (1.0mmol, 162mg), CuI (0.1mmol, 20.0mg), K 3 PO 4 (2.0 mmol, 424.0 mg), and finally 2 mL of DMF was added as a solvent, the reactor was sealed, and the mixture was stirred at 120° C. for 24 h. After the reaction system was cooled, 5 mL of water was added to quench the reaction, and 15 mL of ethyl acetate was used to extract three times. The filtrates were combined and washed twice with water and once with saturated brine. Magnesium sulfate was added to dry for 30 minutes, filtered, and the filtrate was concentrated by rotary evaporation to obtain a crude product. The crude product was separated by column (200-300 mesh silica gel) with a mixture of petroleum ether: ethyl acetate = 12:1 (v / v) as eluent to obtain a yellow solid product 3-phenyliso Quinolinone 115.0mg, isolated yield 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com