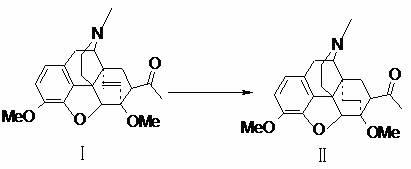
Preparation method of 7alpha-acetyl-6,14-ethyl bridge tetrahydrothebaine
A technology of tetrahydrothebaine and acetyl group, which is applied in the field of double bond hydrogenation reaction of thienorphine intermediates, can solve the problems of low product conversion rate, inconvenient operation, and high equipment requirements, and achieve low investment cost and universal equipment Good performance and high benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Put 14L of absolute ethanol and 1Kg of thienorphine intermediate 7ɑ-acetyl-6,14-ethylene bridge tetrahydrothebaine into a 20L hydrogenation reaction tank, then add 0.2Kg of 10% palladium carbon and 50mL of pyridine, and turn on hydrogen To a pressure of 0.01MPa, first vacuum the system and replace it with hydrogen. Under the condition of hydrogen, stir and heat up to 56°C for hydrogenation reduction reaction for 8 hours. During the reaction, a small amount of fresh hydrogen is exchanged. TLC detects that the reaction is complete. The reduction reaction solution is filtered hot, the filter cake is washed with ethanol, the obtained filtrate is concentrated to near dryness under reduced pressure, and the concentrated product is added with ethanol equivalent to 0.5 times the amount of 7ɑ-acetyl-6,14-ethylene bridged tetrahydrothebaine After stirring evenly, cool to about 5°C, let stand for more than 4 hours and centrifuge, and then wash the centrifuged product with ethanol e...
Embodiment 2
[0030] Put 280mL of anhydrous methanol and 20g of thienorphine intermediate 7ɑ-acetyl-6,14-ethylene bridge tetrahydrothebaine into a 500mL three-necked hydrogenation reaction bottle, then add 4g of 10% palladium carbon and 1mL of pyridine, and turn on hydrogen To a pressure of 0.01MPa, first vacuum the system and replace it with hydrogen. Under the condition of hydrogen, stir and heat up to 56°C for 12 hours. During the reaction, a small amount of fresh hydrogen is exchanged. TLC detects that the reaction is complete. Filtrate hot, wash the filter cake with methanol, concentrate the obtained filtrate to near dryness under reduced pressure, and then use ethanol for 3 times to remove the methanol, and then add the equivalent of 7ɑ-acetyl-6,14-ethylene bridged tetrahydrodiba to the concentrated product Because the ethanol with a dosage of 1.0 times is stirred evenly, cooled to about 5°C, left to stand for more than 4 hours and centrifuged, and the centrifuged product is then used ...
Embodiment 3
[0032] Put 280mL of absolute ethanol and 20g of thienorphine intermediate 7ɑ-acetyl-6,14-ethylene bridge tetrahydrothebaine into a 500mL three-necked hydrogenation reaction bottle, then add 2g of platinum dioxide and 1mL of pyridine, and turn on the hydrogen to The pressure is 0.01MPa, first vacuumize the system and replace it with hydrogen, under the condition of hydrogen, stir and heat up to 56°C for 12 hours, a small amount of fresh hydrogen is exchanged during the reaction, TLC detects that the reaction is complete, first heat the hydrogenation reduction reaction solution Filtrate, wash the filter cake with ethanol, concentrate the obtained filtrate to near dryness under reduced pressure, add ethanol equivalent to 0.7 times the amount of 7ɑ-acetyl-6,14-ethylene-tetrahydrothebaine, stir evenly, and cool to 5 Centrifuge at about 4 hours, the centrifuged product is then washed with ethanol equivalent to about 1 times the amount of 7ɑ-acetyl-6,14-ethylene bridged tetrahydrotheb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com