Fire retardant containing boron phenyl phosphonic acid esters and preparation method thereof
A technology of boron phenyl phosphonate and flame retardant, which is applied in the field of preparation of boron-containing phenyl phosphonate flame retardant, which can solve the problems of low flame retardant efficiency, reduced impact performance of polymer materials, inconvenient processing and use, etc. problem, to achieve the effect of high flame retardancy and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
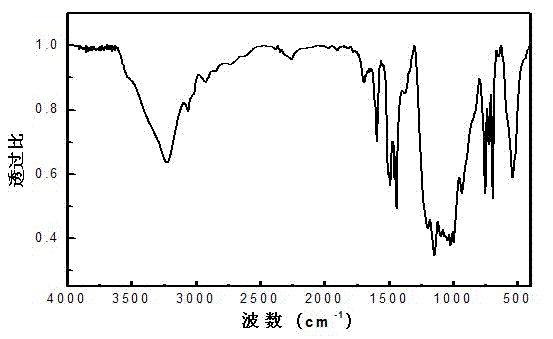
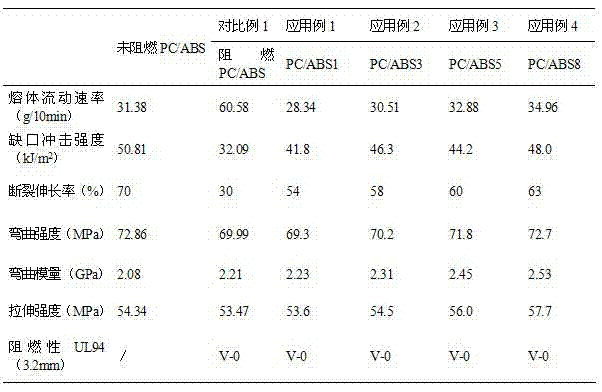
Image
Examples
Embodiment 1
[0020] Add 0.30mol o-hydroxybenzyl alcohol and 90ml toluene into a 250ml three-necked flask equipped with a mechanical stirrer and a water separator, heat up to 90°C, add 0.09mol boric acid, then heat the system to a boiling state and react for 50 minutes, then cool , remove unreacted boric acid and o-hydroxybenzyl alcohol by filtration to obtain a hydroxybenzyl borate intermediate; add all the hydroxybenzyl borate intermediates to a spherical Add 0.30 mol of phenylphosphoryl dichloride and 60 ml of toluene to a 250ml four-neck flask connected to nitrogen with a condenser tube and an elbow connection, protect with nitrogen, react at 25°C for 12 hours, and remove excess phenylphosphoryl dichloride under reduced pressure. Chlorine and toluene; replace the decompression device with a reflux device, add 0.30mol phenol, react at 120°C for 2 hours, and then react at 150°C for 4 hours, remove excess phenol under reduced pressure, and place the resulting product in a vacuum oven at 60°...
Embodiment 2
[0024] Add 0.25mol p-hydroxybenzyl alcohol and 150ml xylene into a 500ml three-necked flask equipped with a mechanical stirrer and a water separator, heat up to 80°C, add 0.125mol trimethyl borate, and then heat the system to a boiling state for reaction For 40 minutes, cool, filter and remove boric acid and unreacted hydroxybenzyl alcohol to obtain hydroxybenzyl borate intermediate; add all the hydroxybenzyl borate intermediate to a machine equipped with a mechanical stirrer and connect the hydrogen chloride absorption through an elbow joint. Into a 500ml four-neck flask connected to nitrogen with the spherical condenser and the elbow of the device, add 0.50mol phenylphosphoryl dichloride, 1.46g AlCl 3 And 100ml of xylene, under nitrogen protection, react at 50°C for 8 hours, remove excess phenylphosphoryl dichloride and xylene under reduced pressure; replace the decompression device with a reflux device, add 1.0mol phenol, and react at 130°C After 2 hours and 3 hours at 160°...
Embodiment 3
[0028] Add 0.30mol of m-hydroxybenzyl alcohol and 120ml of 1,4-dioxane into a 500ml three-necked flask equipped with a mechanical stirrer and a water separator, heat up to 85°C, add 0.18mol of triethyl borate, and then put the system Heated to boiling state and reacted for 60 minutes, cooled, filtered to remove boric acid and unreacted hydroxybenzyl alcohol to obtain hydroxybenzyl borate intermediate; Add 0.45mol phenylphosphoryl dichloride, 1.32g MgCl 2And 180ml 1,4-dioxane, under nitrogen protection, react at 75°C for 5 hours, remove excess phenylphosphoryl dichloride and 1,4-dioxane under reduced pressure; change the decompression device to reflux device, add 0.8mol phenol, react at 125°C for 4 hours, and then react at 160°C for 2 hours, remove excess phenol under reduced pressure; dissolve the product in tetrahydrofuran, filter to remove MgCl 2 Afterwards, THF was distilled off by heating, and the obtained product was dried in a vacuum oven at 80°C to obtain a boron-conta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| impact strength | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com