Isopentene flavonol glycoside derivative and preparation method and application thereof
A technology for isopentenyl flavonol glycosides and derivatives, which is applied in the field of medicine and can solve the problems of high price, uncertain long-term efficacy, large toxic and side effects, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
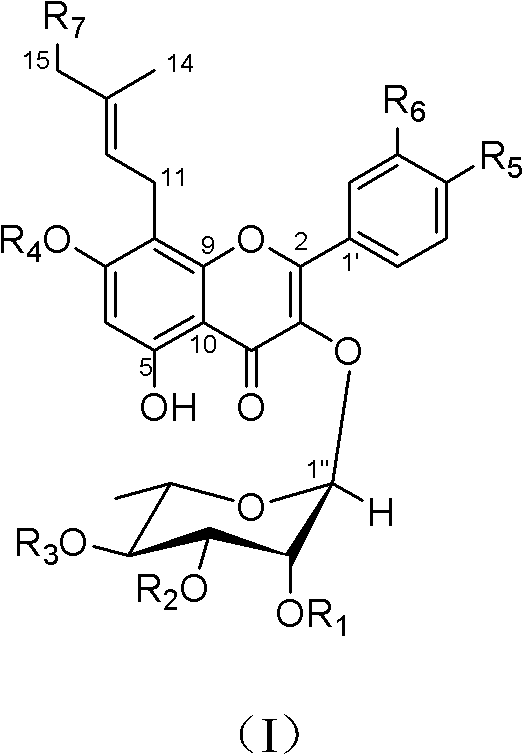
[0055] Embodiment 1: Preparation of prenyl flavonol glycoside derivatives of the present invention
[0056] 1. Take 2 kg of dry Epimedium pilosa aerial parts (aerial parts except the root diameter, etc.), heat and reflux extract 2 times with 60% ethanol of 8 times the amount, each time for 2 hours, and the extract is evaporated to dryness under reduced pressure Afterwards, extract 245g is obtained;
[0057] 2. Suspend the extract in 5L of water, filter it, put the filtrate on a macroporous adsorption resin HP-20 chromatographic column (Ф9×70cm), and wash it with water, 30%, 95% (v / v) ethanol-water in sequence , wherein, 95% (v / v) ethanol-water elution part obtains 80g altogether;
[0058] 3. Separate 80 g of 95% (v / v) ethanol-water eluted part with silica gel open column chromatography, adopt chloroform-methanol solvent system gradient elution, and collect the eluent with each 1 / 3 column retention volume as a part, The eluents were combined according to the different spots o...
experiment example 2
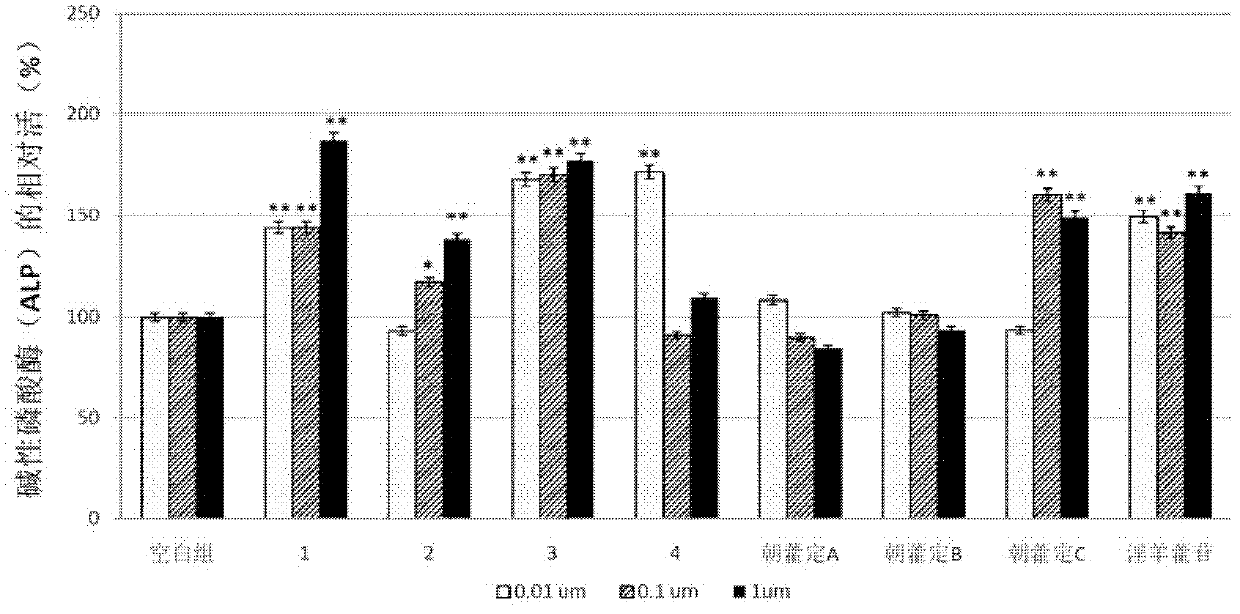
[0085] Experimental Example 2: Effects of the compounds and analogs obtained in Example 1 on the alkaline phosphatase activity (ALP) of the osteoblast-like cell line MC3T3-E1 cells.
[0086] With the ALP activity of osteoblast-like cell line MC3T3-E1 cells, evaluate the promotion of osteoblasts by the compounds obtained in Example 1 and structural analogues Epimedin A, Epimedin B, Epimedin C and Icariin The role of differentiation.
[0087] MC3T3-E1 cells were seeded in 24-well plates (1×10 5 cells / well), cultured in α-MEM medium containing 10% FBS (fetal bovine serum) (37°C, humidity 95%, 5% CO 2 ).
[0088] After 2 days, the MC3T3-E1 cells were replaced with α-MEM medium containing 0.3% FBS and different concentrations of compounds (0.01 μmol / L, 0.1 μmol / L, 1 μmol / L). Cultured in α-MEM medium with %FBS as the blank group, continued to culture for 10 days.
[0089] After the culture, discard the original culture medium, fix the cells with 0.2% Nonidet P-40 (2 mL), and aft...
experiment example 3
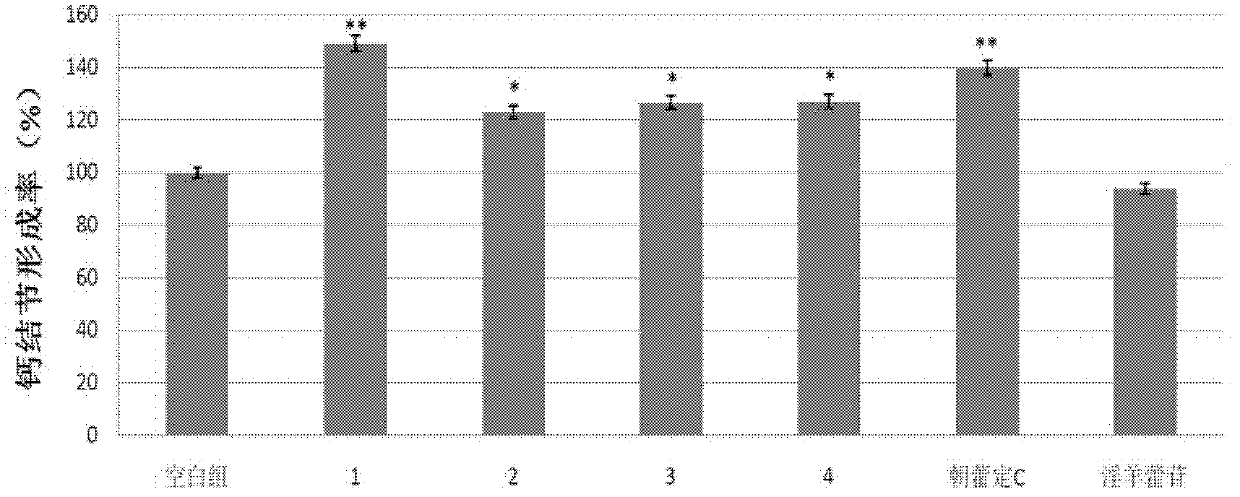
[0092] Experimental example 3: Effect on the promotion rate of calcium nodule formation of osteoblast-like cell line MC3T3-E1 cells
[0093] With the calcium nodule formation rate of osteoblast-like cell line MC3T3-E1 cells, evaluate the activity of promoting osteoblast calcification of the compound obtained in Example 1 and structural analogs epimedin C and icariin, the specific method is :
[0094] MC3T3-E1 cells were seeded in 12-well plates (1×10 5 cells / well), cultured in α-MEM medium containing 10% FBS (37°C, humidity 95%, 5% CO 2 ).
[0095] Two days later, the cells were replaced with α-MEM medium containing 1 μmol / L compound or blank solvent, and 10 mmol / L β-glycerophosphate sodium and 50 μg / mL ascorbic acid solution were added to induce mineralization.
[0096] The culture medium was replaced every other day, and the culture was terminated after 12 days. Alizarin red staining was used for morphometric analysis. Taking the mineralized area (A) as an index, calcula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com