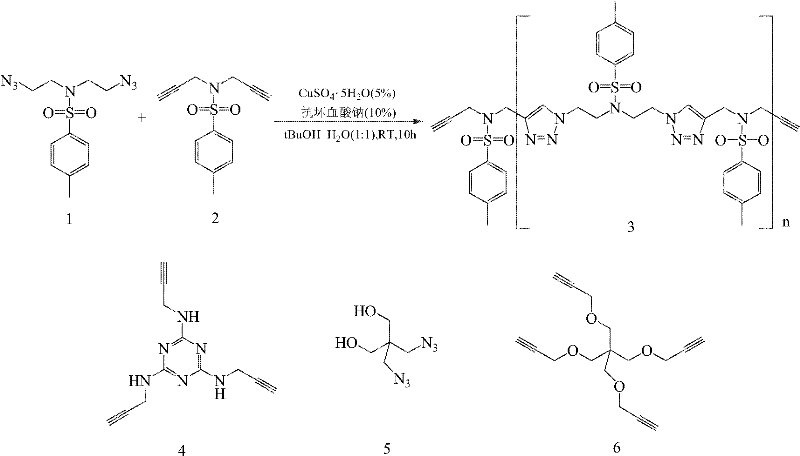
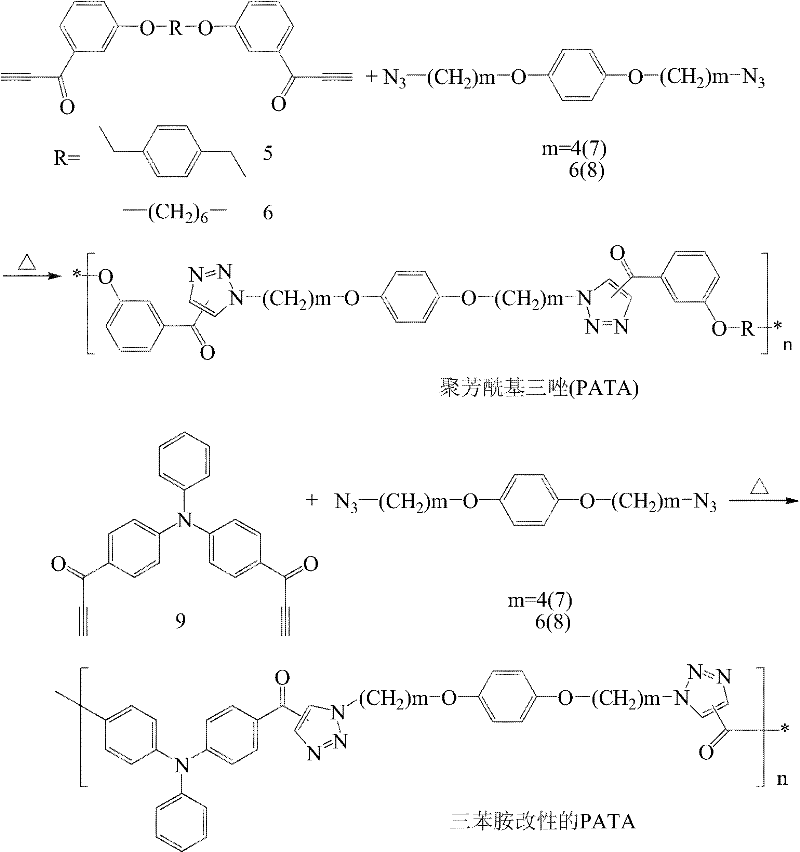
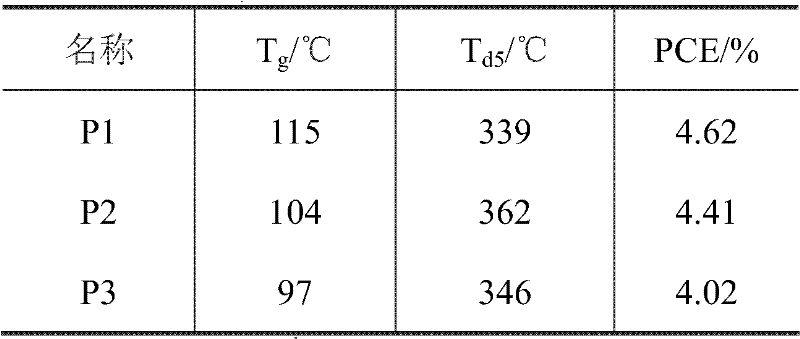
Novel polytriazole resins with rigid structure and preparation method thereof
A technology of polytriazole resin and rigid structure, applied in the field of resin, can solve problems such as no research reports, and achieve the effect of good mechanical properties and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of PATA-1
[0040] (1) Synthesis of 4,4' diazide diphenylmethane
[0041]Add 210mL of deionized water and 120mL of concentrated hydrochloric acid to a 1000mL four-necked round-bottomed flask containing 5.94g (30mmol) of 4,4'diaminodiphenylmethane, and stir to control the temperature of the mixture at 0-5°C. Add 90mL of an aqueous solution containing 6.18g (90mmol) of sodium nitrite, which turns light yellow, and the reactant is gradually homogeneous under constant stirring, turning light yellow or white. After stirring for 1 hour, the temperature is still controlled at 0-5°C, and then added dropwise 90mL of an aqueous solution containing 4.86g (75mmol) of sodium azide, the system was light red, and nitrogen gas was observed to be generated, the color was gradually brownish yellow, and precipitation occurred, and the stirring was stopped after 6h, and the reactant was allowed to stand for 12h, filtered, filtered The cake was washed with deionized water and d...
Embodiment 2
[0046] Preparation of PATA-2
[0047] (1) Synthesis of 4,4'-diazide diphenyl ether
[0048] Add 210mL of deionized water and 120mL of concentrated hydrochloric acid to a 1000mL four-necked round-bottomed flask containing 6.00g (30mmol) of 4,4'diaminodiphenyl ether, stir, and control the temperature of the mixture at 0-5°C, drop Add 90mL of an aqueous solution containing 6.18g (90mmol) of sodium nitrite, which turns light yellow, and the reactant is gradually homogeneous under constant stirring, turning light yellow or white. After stirring for 1 hour, the temperature is still controlled at 0-5°C, and then added dropwise 90mL of aqueous solution containing 4.86g (75mmol) sodium azide, the system was yellow, and it was observed that nitrogen gas was generated, the color was gradually brownish yellow, and precipitation appeared. Stirring was stopped after 6 hours, and the reactant was left to stand for 12 hours, filtered, and the filter cake was washed with deionized water, and ...
Embodiment 3
[0053] Preparation of PATA-3
[0054] (1) Synthesis of 4,4'-diphenylsulfone diazide
[0055] Add 210mL of deionized water and 120mL of concentrated hydrochloric acid to a 1000mL four-necked round-bottomed flask containing 7.44g (30mmol) of 4,4'diaminodiphenylsulfone, and stir to control the temperature of the mixture at 0-5°C. Add 90mL of aqueous solution containing 6.18g (90mmol) sodium nitrite, it is light yellow, and the reactant is gradually homogeneous under continuous stirring, and it is light yellow or white. After stirring for 1 hour, the temperature is still controlled at 0~5℃, and then add 90mL of aqueous solution containing 4.86g (75mmol) sodium azide, the system was white, and it was observed that nitrogen gas was generated, the color gradually became light yellow, and precipitation appeared. Stirring was stopped after 6 hours, and the reactant was left to stand for 12 hours, filtered, and the filter cake was washed with deionized water, and dried to obtain a ligh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
| Shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com