Progesterone ethosome, and preparation method and application thereof
A progesterone and ethosome technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the limitations of the wide application of progesterone transdermal drug delivery system, cumulative penetration and penetration rate Low, no progesterone ethosomes reported, to achieve the effect of improving bioavailability and therapeutic index, reducing drug dosage and side effects, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
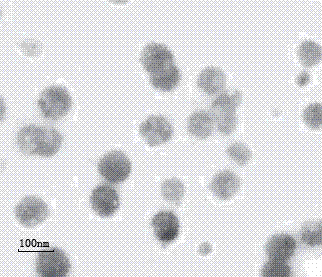
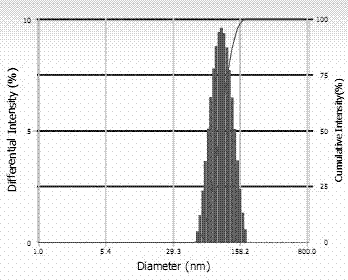
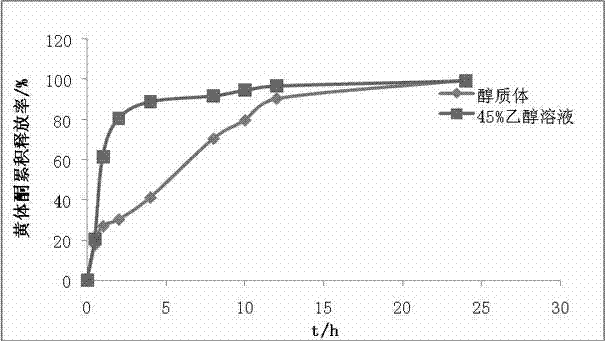
Image
Examples
Embodiment 1
[0049]The percentage in the prescription is the weight share ratio of this component in the whole prescription, and the following examples are the same.
[0050] Progesterone 0.125%
[0052] Cholesterol 0.4%
[0053] Tween-80 1%
[0054] Ethanol 30%
[0055] The balance is deionized water
[0056] The preparation method step of progesterone ethosome is as follows:
[0057] Step 1: Fully mix and dissolve progesterone, egg yolk lecithin, cholesterol and absolute ethanol to form an alcohol phase; fully mix Tween-80 and deionized water to form a water phase;
[0058] Step 2: Heat the water phase and the oil phase to 40°C respectively, keep the temperature constant, add the alcohol phase to the water phase under stirring conditions, and make colostrum;
[0059] Step 3: Homogenize colostrum high-pressure milk to obtain a suspension;
[0060] Step 4: Stir the ethosome suspension for 30 minutes and perform secondary emulsification;
[0061] Step 5: ...
Embodiment 2
[0068] The percentage in the prescription is the weight share ratio of this component in the whole prescription, and the following examples are the same.
[0069] Progesterone 0.6%
[0071] Cholesterol 0.4%
[0072] Tween-80 1%
[0073] Ethanol 30%
[0074] The balance is deionized water
[0075] The preparation method step of progesterone ethosome is as follows:
[0076] Step 1: Fully mix and dissolve progesterone, egg yolk lecithin, cholesterol and absolute ethanol to form an alcohol phase; fully mix Tween-80 and deionized water to form a water phase;
[0077] Step 2: Heat the water phase and the oil phase to 40°C respectively, keep the temperature constant, add the alcohol phase to the water phase under stirring conditions, and make colostrum;
[0078] Step 3: Homogenize colostrum high-pressure milk to obtain a suspension;
[0079] Step 4: Stir the ethosome suspension for 15 minutes for secondary emulsification;
[0080] Step 5: The suspe...
Embodiment 3
[0083] The percentage in the prescription is the weight share ratio of this component in the whole prescription, and the following examples are the same.
[0084] Progesterone 0.2%
[0086] Tween-80 0.5%
[0087] Propylene Glycol 30%
[0088] The balance is deionized water
[0089] The preparation method step of progesterone ethosome is as follows:
[0090] Step 1: Fully mix and dissolve progesterone, egg yolk lecithin, and propylene glycol to form an alcohol phase; fully mix Tween-80 and deionized water to form a water phase;
[0091] Step 2: Heat the water phase and the oil phase to 30°C respectively, keep the temperature constant, add the alcohol phase to the water phase under stirring conditions, and make colostrum;
[0092] Step 3: Homogenize colostrum high-pressure milk to obtain a suspension;
[0093] Step 4: Stir the ethosome suspension for 20 minutes for secondary emulsification;
[0094] Step 5: The suspension is placed at 0-4°C, cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com