Patents
Literature
33 results about "Premenstrual syndrome (PMS)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
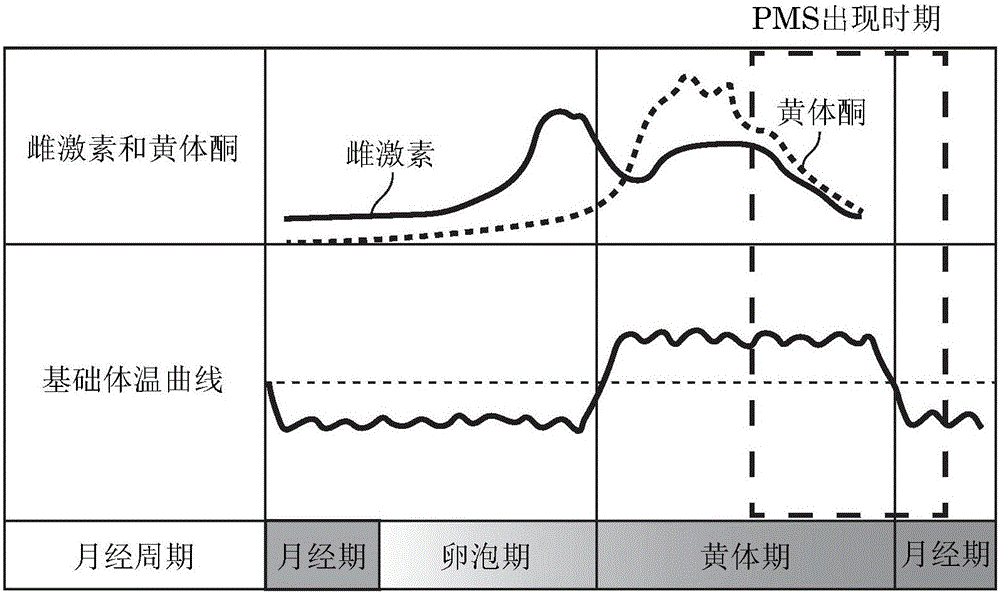
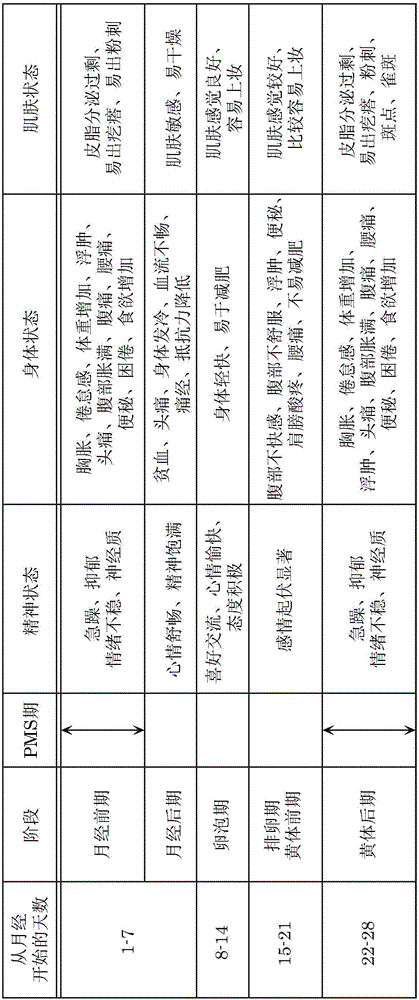
Physical and emotional symptoms experienced days before a woman's period.
Composition comprising cocoa
InactiveUS20040005347A1Improve obesityReducing appetite and carbohydrate cravingBiocideOrganic active ingredientsDecreased LibidoAmine receptor
The invention pertains to a composition and a method for the treatment of mood disorders, in particular of treating, preventing or alleviate depression, mood disorders or insufficient mood, obesity, overweight, premenstrual syndrome, craving, carbohydrate craving, chocolate craving, menopausal complaints, erectile dysfunction and / or reduced libido. The composition contains cocoa or one or more of its pharmacologically active components, and a dopamine D2 receptor agonist.
Owner:NV NUTRICIA
Combination of serotonin reuptake inhibitors and norephinephrine reuptake inhibitors
InactiveUS20050014848A1Prevent relapseIncreasing and improving neuronal processBiocideAmine active ingredientsStress inducedNorepinephrine reuptake inhibitor
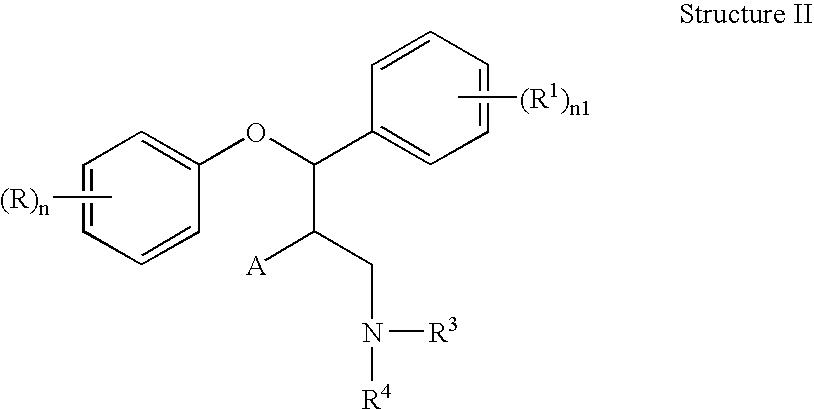
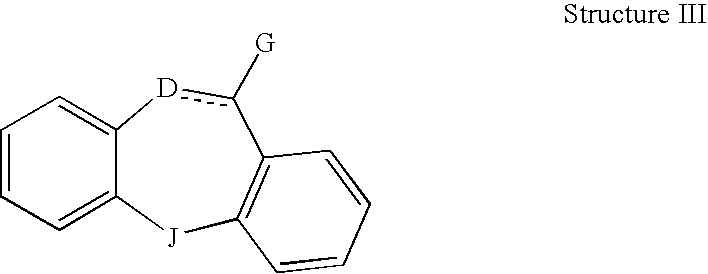
This invention is directed to pharmaceutical compositions and methods for treating a disorder or condition selected from the group consisting of depression, anxiety disorders, phobias, avoidant personality disorder, eating disorders, chemical dependencies, Parkinson's diseases, obsessive-compulsive disorder, negative symptoms of schizophrenia, cognitive dysfunction related to schizophrenia, premenstrual syndrome, stress-induced incontinence, headache, neuropathic pain, chronic pain, urinary incontinence, post-traumatic stress disorder, chronic stress, acute stress, fibromyalgia, depression comorbid with fibromyalgia, obesity, migraine and a combination thereof in a mammal. The methods in one embodiment comprise administering to a mammal in need of treatment for the disorder or condition: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined in the specification; and (iii) a pharmaceutically acceptable carrier. The pharmaceutical compositions and methods of the invention are also useful for preventing a relapse associated with one of the foregoing disorders or conditions, and for treating a symptom associated with one of the foregoing disorders or conditions, wherein the symptom is selected from the group consisting of cognitive dysfunctions and somatic complaints.
Owner:PFIZER INC
Compositions containing policosanol and chromium and/or chromium salts and their pharmaceutical uses
InactiveUS20060024383A1Increasing lean body massLower blood sugar levelsHeavy metal active ingredientsBiocideCoronary heart diseaseHypoglycemia
A composition is provided which contains policosanol and chromium and / or chromium salts and which may be used for treating, preventing and or reducing metabolic syndrome, hypercholesterolemia and hypoglycemia related diseases, total cholesterol, LDL-cholesterol, LDL / HDL ratio, triglycerides, coronary heart disease (heart attacks and strokes), inflammation, deep-vein thrombosis, immunoregulatory diseases, cardiovascular diseases, obesity, insulin resistance, dyslipidemia, raised blood pressure, fatigue, premenstrual syndrome, anxiety, depression and / or neurodegenerative disorders, and / or raising HDL cholesterol and / or lean body mass in humans and animals. The method comprises administering policosanol and chromium and / or chromium salts which together effectively lower blood glucose levels and lower the LDL / HDL cholesterol ratio. Typically, the administered composition includes about 0.1-10:1 parts by weight of policosanol to chromium and / or chromium salts.
Owner:WYETH
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Condensed-ring thiophene derivatives, their production and use
A gonadotropin-releasing hormone antagonistic composition, which comprises an optionally substituted condensed-bicyclic compound consisting of a homo or hetero 5 to 7 membered ring and a homo or hetero 5 to 7 membered ring is effective as a propylactic or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, cancer of uterine cervix, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; is effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a contraceptive of male or female, as an ovulation-inducing agent of female; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; is useful as modulating estrous cycles in animals in the field of animal husbandry, as an agent fro improving the quality of edible meat or promoting the growth of animals; is useful as an agent of spawning promotion in fish.
Owner:TAKEDA PHARMA CO LTD
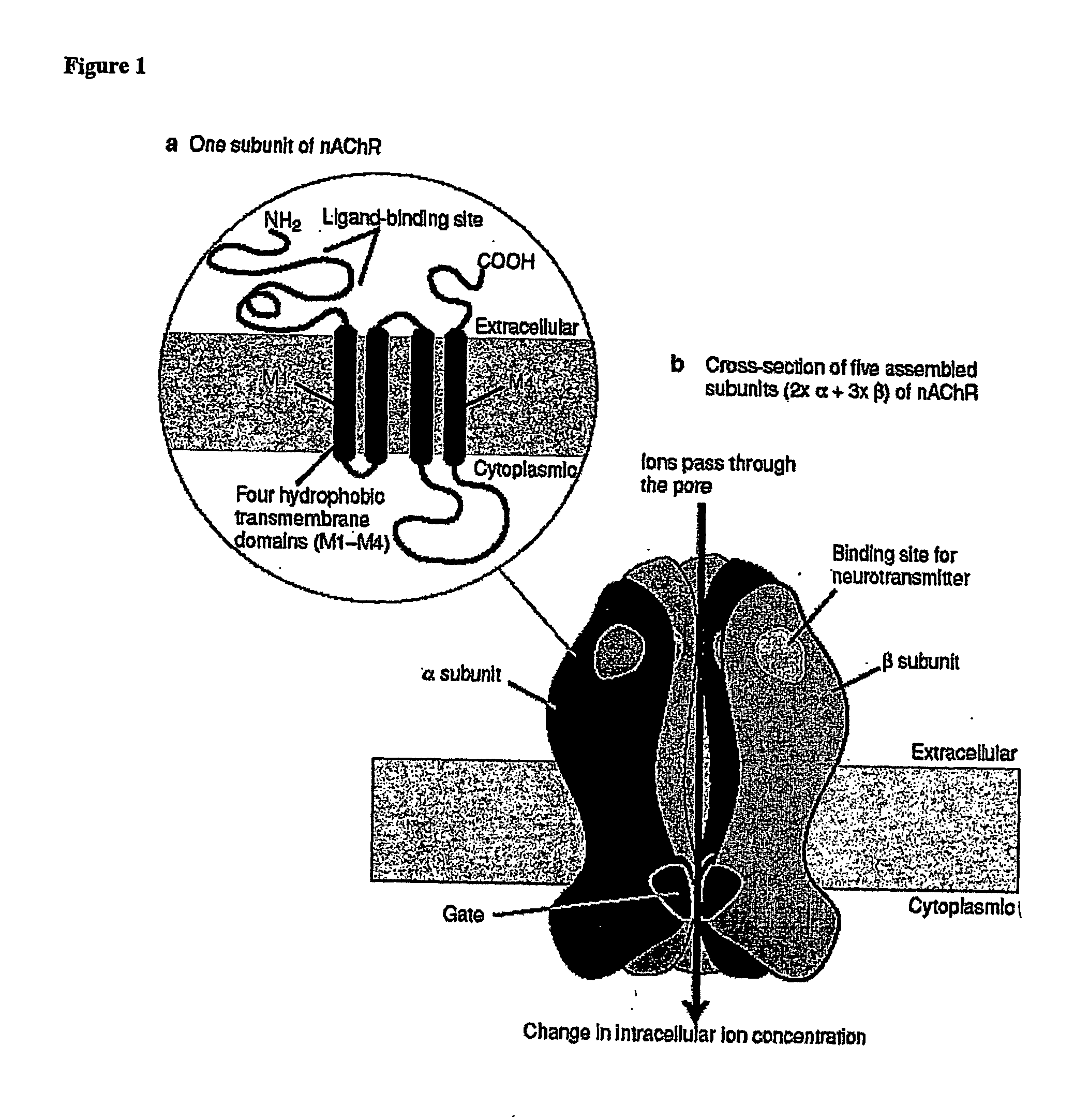
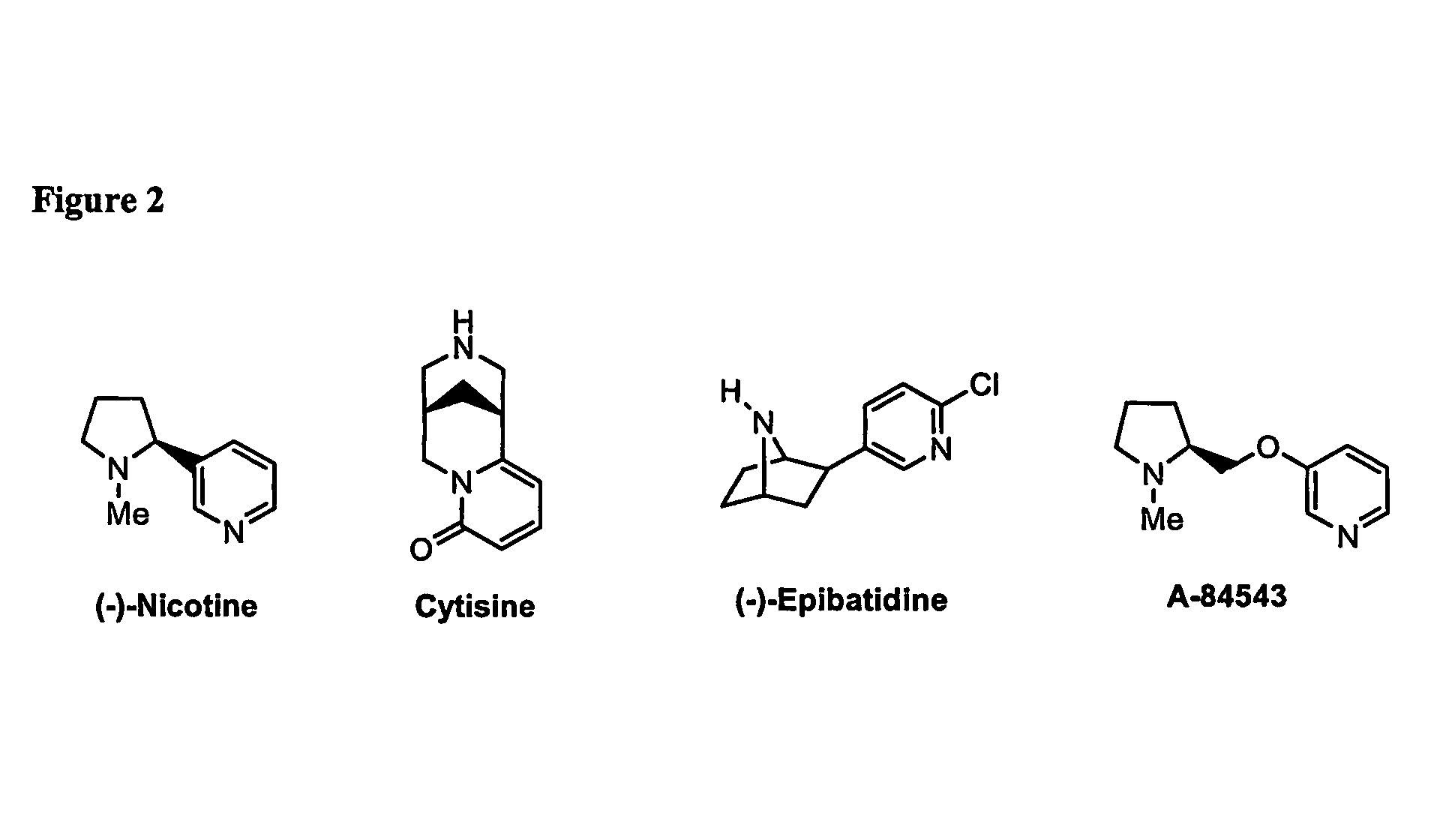
Ligands for Nicotinic Acetylcholine Receptors, and Methods of Making and Using Them
ActiveUS20080132486A1Easily determining tailoringSimple designBiocideNervous disorderDiseaseAlcoholisms
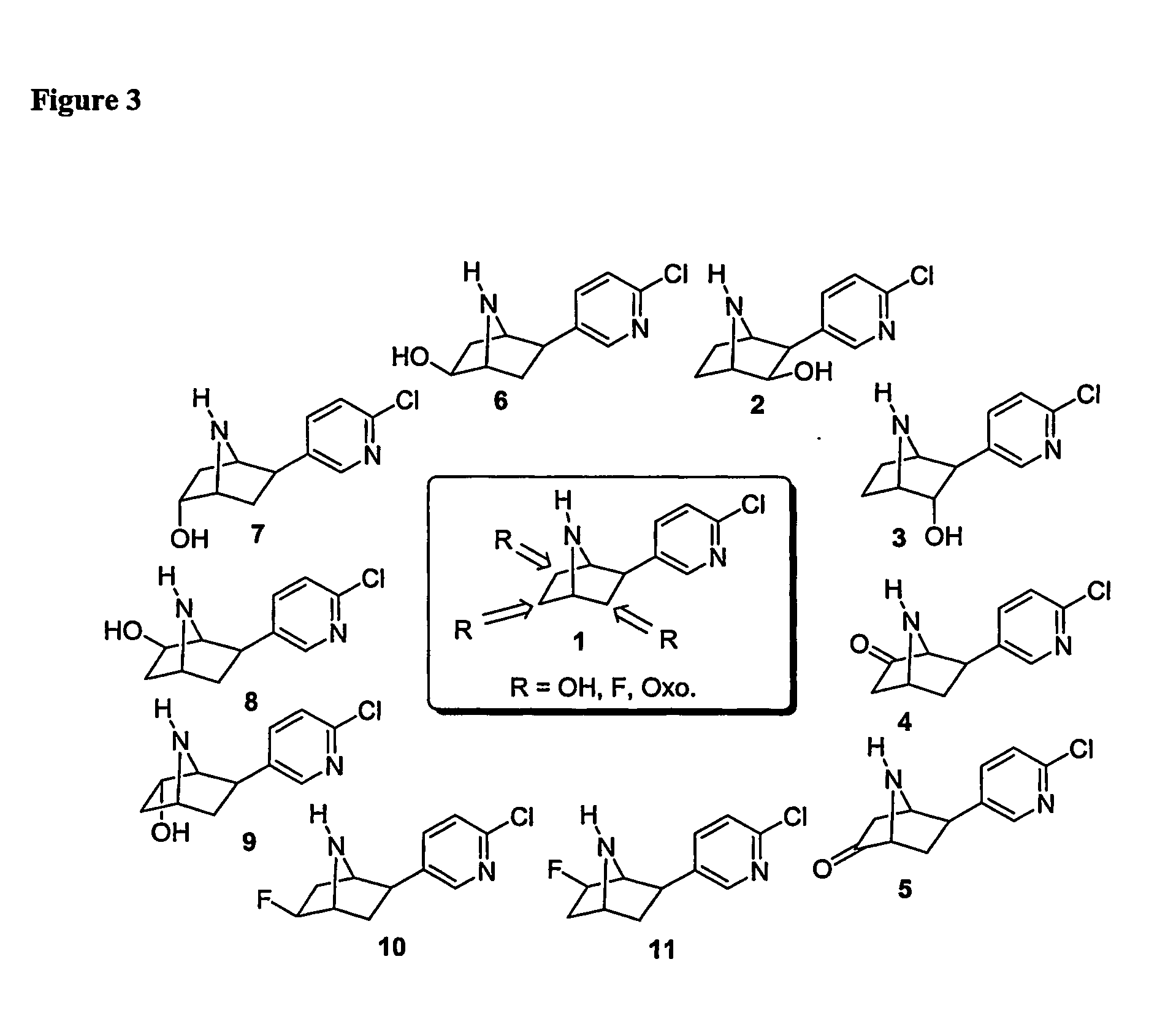
One aspect of the present invention relates to heterocyclic compounds that are ligands for nicotinic acetylcholine receptors. A second aspect of the invention relates to the use of a compound of the invention for modulation of a mammalian nicotinic acetylcholine receptor. The present invention also relates to the use of a compound of the invention for treating a mammal suffering from Alzheimer's disease, Parkinson's disease, dyskinesias, Tourette's syndrome, schizophrenia, attention deficit disorder, anxiety, pain, depression, obsessive compulsive disorder, chemical substance abuse, alcoholism, memory deficit, pseudodementia, Ganser's syndrome, migraine pain, bulimia, obesity, premenstrual syndrome or late luteal phase syndrome, tobacco abuse, post-traumatic syndrome, social phobia, chronic fatigue syndrome, premature ejaculation, erectile difficulty, anorexia nervosa, disorders of sleep, autism, mutism or trichtillomania.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Preparation for relieving stress symptom
The present invention relates to a preparation mainly for preventing and curing the diseases of tonicity, dysphoria, lassitude, light-headedness, hypomnesis and female premenstrual syndrome. It is characterized by that said preparation contains gamma-aminobutyric acid and theanine. Besides, in said preparation the medicinal plant, amino acid, vitamin, mineral and auxiliary material which can be used as additive in food or medicine also can be added.
Owner:郭凌云
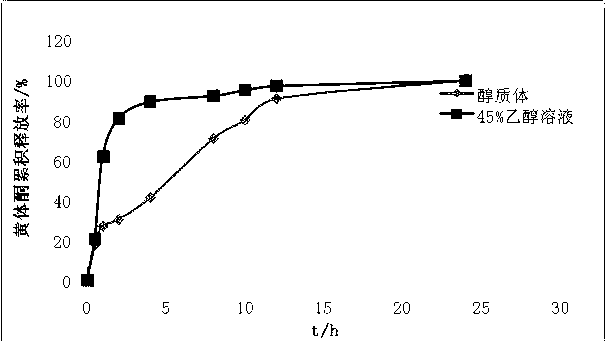
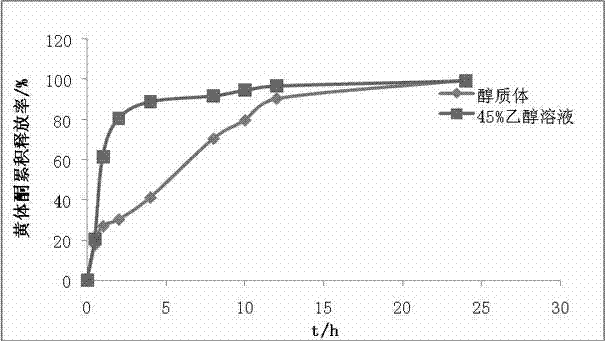
Progesterone ethosome, and preparation method and application thereof
ActiveCN102397255AImprove stabilityReduced stabilityOrganic active ingredientsSexual disorderCholesterolActive agent
The invention provides a progesterone ethosome. Progesterone is encapsulated in an ethosome. The progesterone ethosome comprises the following components in percentage by weight: 0.1 to 1 percent of progesterone, 1 to 8 percent of lipid materials, 0 to 0.6 percent of cholesterol, 20 to 50 percent of short-chain alcohols, 0 to 3 percent of nonionic surfactant and the balance of water. The preparation method comprises the following steps of: mixing and dissolving the progesterone, the lipid materials, the cholesterol and the short-chain alcohols to prepare an alcohol phase; with stirring, adding the alcohol phase into a nonionic surfactant-containing aqueous phase to prepare primary emulsion under the condition of stirring; homogenizing the primary emulsion under a high pressure to obtain asuspension; stirring the ethosome suspension for 15 to 30 minutes to perform emulsification for the second time; and curing the suspension by cooling the suspension at 0 to 4 DEG C to obtain the progesterone ethosome. Because the high-pressure homogenization method is adopted to prepare the progesterone ethosome, the progesterone ethosome is low in stimulation to skin and high in transdermal delivery ability, and metal ion pollution which is easy to cause by the probe ultrasound method of the traditional homogenization method is avoided. Therefore, the progesterone ethosome is suitable for industrial production. The progesterone ethosome can be prepared into a transdermal drug delivery system, a mucosal drug delivery system, and topical dosage forms, such as a paster, a gel and the like. The progesterone ethosome is mainly applied to hormone replacement therapy, secondary amenorrhea, functional aplastic bleeding, premenstrual syndrome and the like clinically.
Owner:GUANGDONG PHARMA UNIV
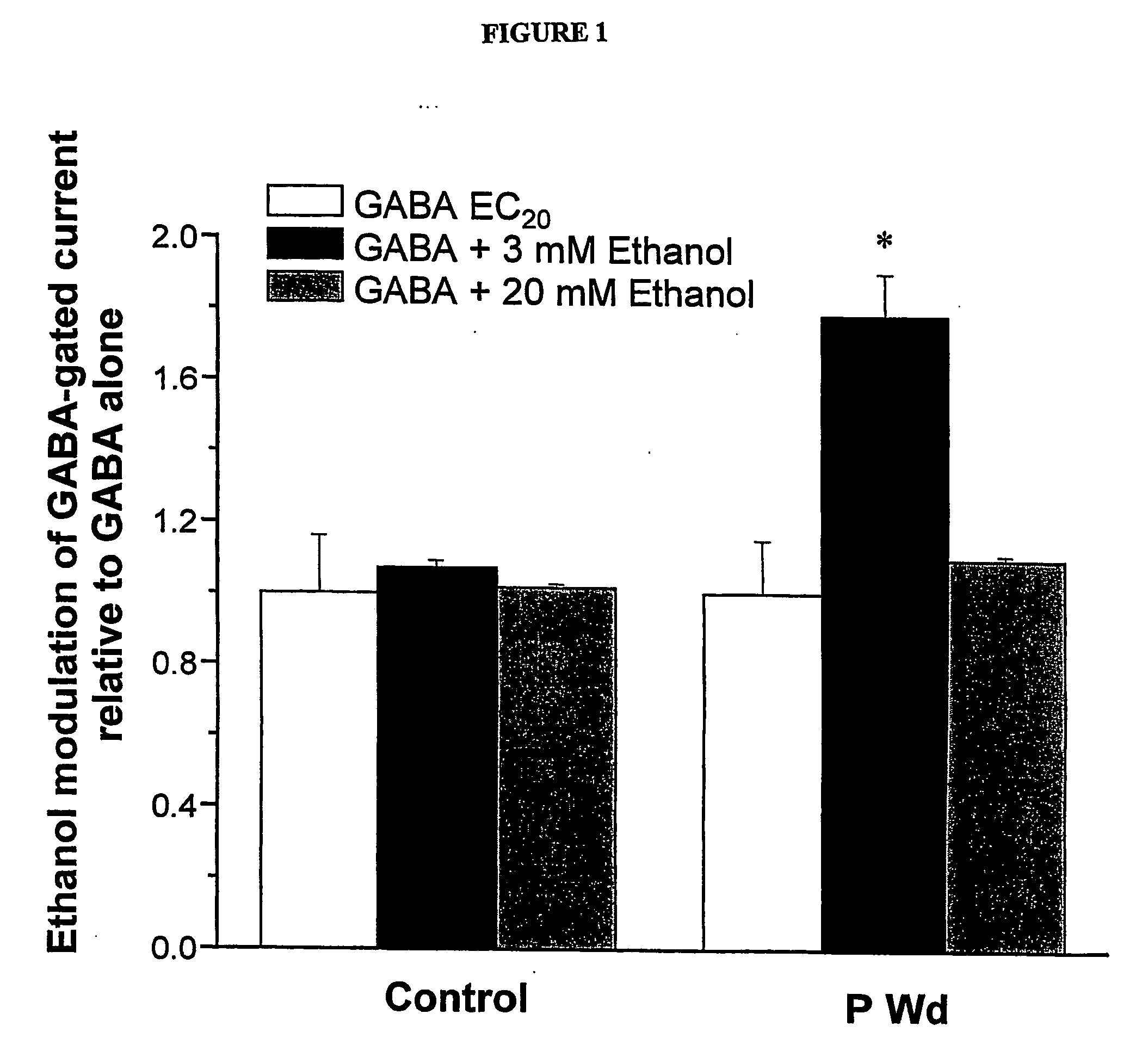
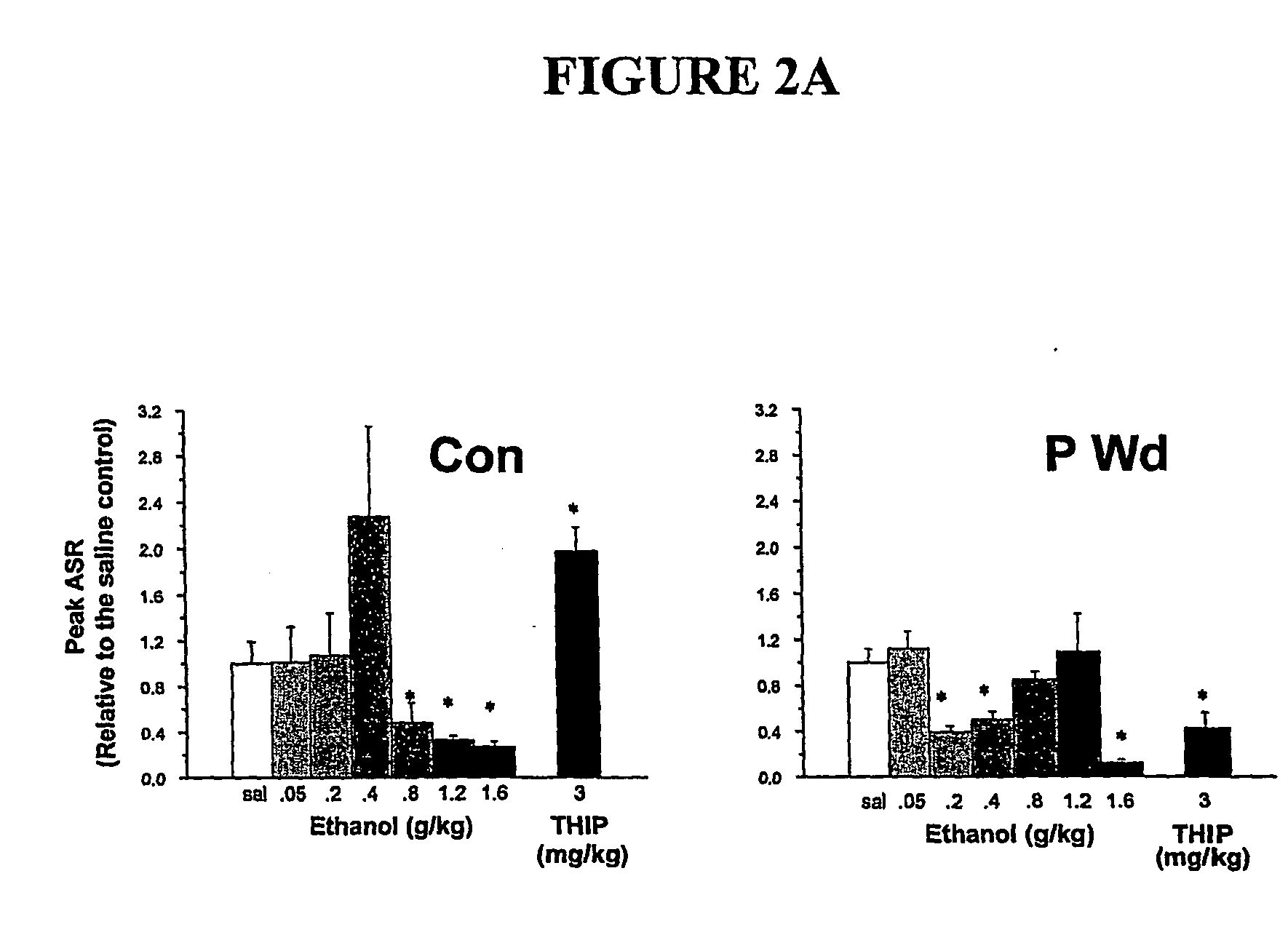
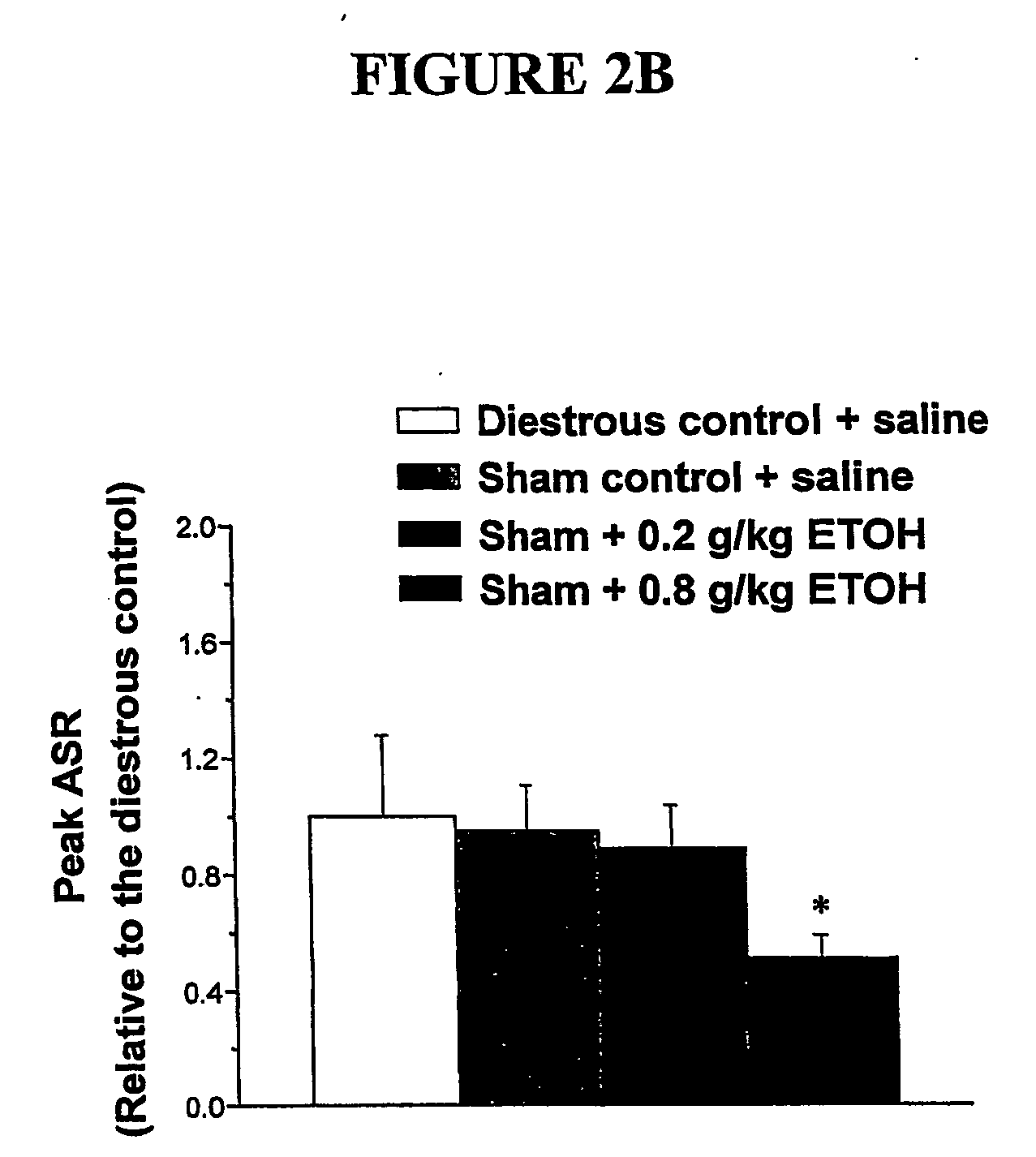
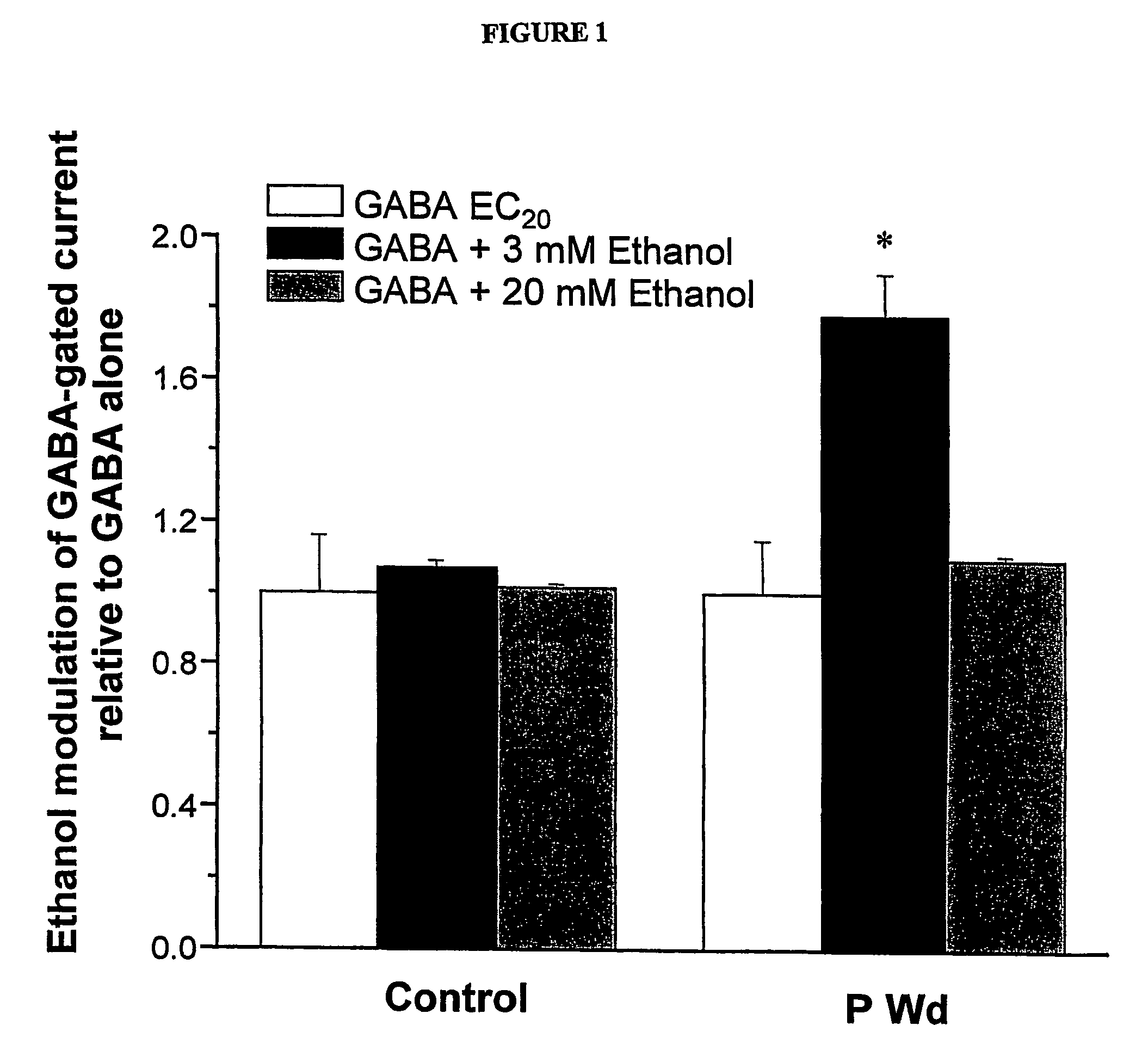
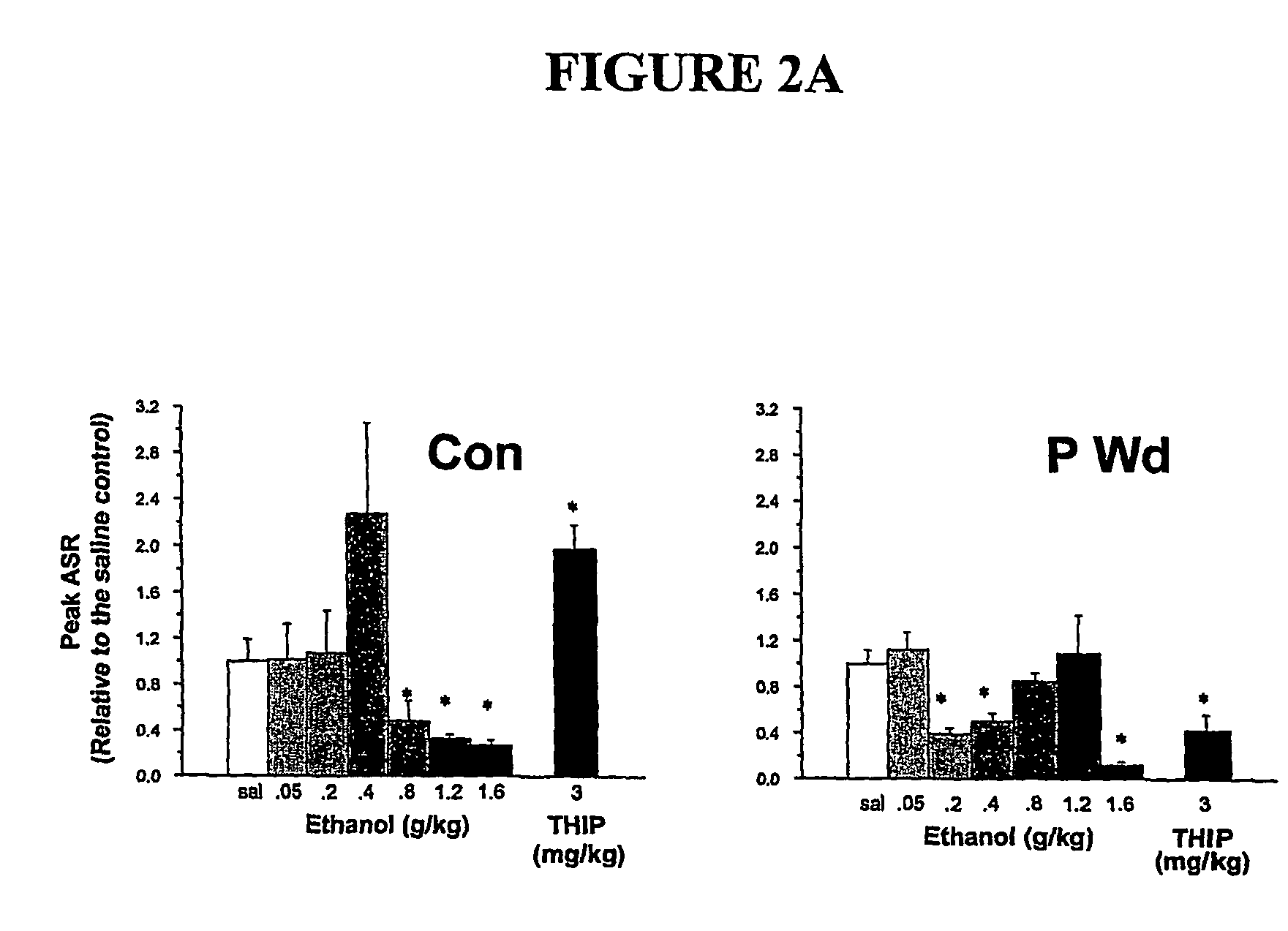
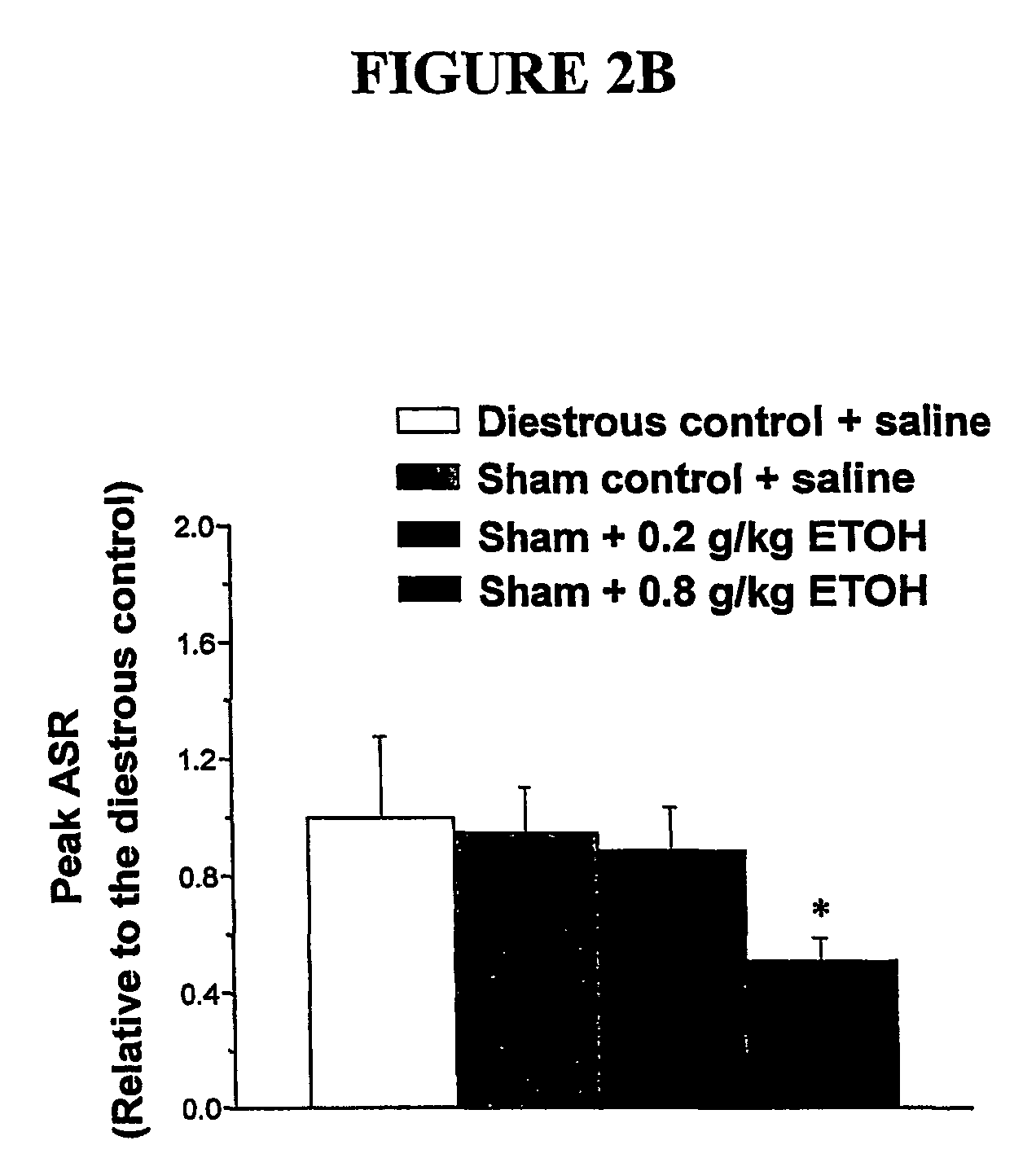
α4 β2 δGABA-A receptors as a strategy for PMS and alcoholism
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Chinese medicine composition for treating menstrual period syndrome
The present invention relatesto a Chinese medicine composition for curing premenstrual syndrome. Said Chinese medicine copmosition is formed from tonifying medicine, medicine for relieving exterior syndrome, medicine for regulating qi, medicine for clearing away heat, medicine for dissipating damp with aromatics and medicine for promoting blood circulation and removing stasis.
Owner:QINHUANGDAO SHANHAIGUAN PHARMA CO LTD
Lighting control system
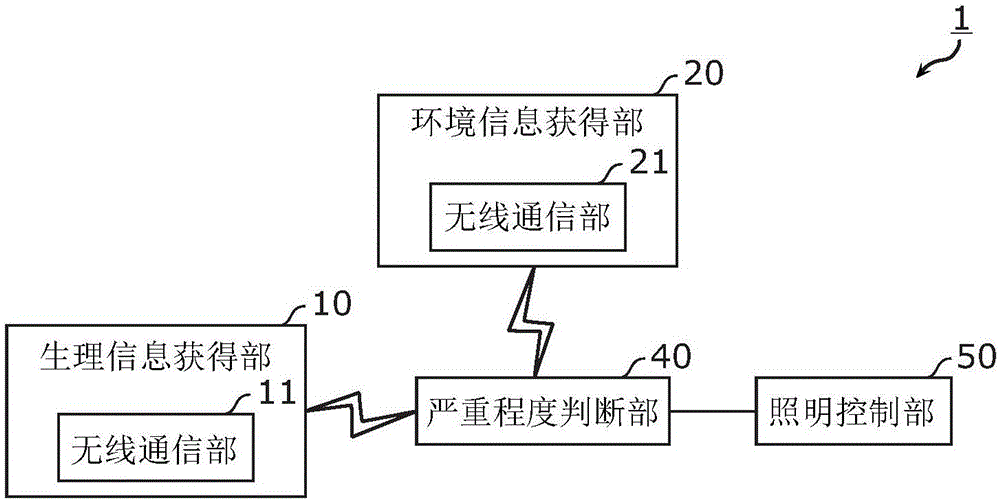
InactiveCN106507561ARelieve symptomsElectrical apparatusVaccination/ovulation diagnosticsBiological bodyEffect light
A lighting control system includes: a biological information obtainer (10) which obtains biological information related to a biological body of a user; an environmental information obtainer (20) which obtains environmental information related to a surrounding environment of the user; a severity determiner (40) which determines a severity of premenstrual syndrome (PMS) based on the biological information obtained by the biological information obtainer (10) and the environmental information obtained by the environmental information obtainer (20); and a lighting controller (50) which controls a lighting device based on the severity determined by the severity determiner (40).
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Vitex trifolia extract and its preparing method and use
InactiveCN1562155AHigh content of active ingredientsQuality controllableUnknown materialsSexual disorderBiotechnologyMethanol water
Two extracts VT-1 and VT-2 of shrub chastetree fruit for treating periodic mastodynia, menalgia and premenstrual syndrome is prepared through extracting in mixture of water and methanol or ethanol, separating by adsorptive resin to remove impurities, and eluting by the aqueous solution of alcohol with different concentrations.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Polymorphic forms of 1-'4-(5-cyanoindol-3-yl)butyl-4-(2-carbamoylbenzofuran-5-yl)piperazine hydrochloride
InactiveCN101139345ALow hygroscopicityEasy to compressOrganic active ingredientsNervous disorderFibromyalgiaPsychotic illness
Owner:MERCK PATENT GMBH
Gel dressing for preventing and inhibiting HPV virus infection and preparation method thereof
InactiveCN111000890AImprove immunityPromote absorptionPeptide/protein ingredientsHydroxy compound active ingredientsCarrageenanGlycerol
The invention discloses a gel dressing for preventing and inhibiting HPV virus infection. The gel dressing is prepared from hydrogel and traditional Chinese medicine extracts uniformly dispersed in the hydrogel, wherein the traditional Chinese medicine extracts include radix sophorae flavescentis extract, scouring rush extract, brucea javanica extract, fructus cnidii extract, anthocyanin, carrageenan, natural equilibrium factors and borneol; and the hydrogel includes carboxymethyl cellulose sodium, ethylparaben, propylene glycol, mannitol, antibacterial peptide, glycerol, a water-based powdered lubricant, diethanol amine and distilled water. By adopting the technical scheme, the gel dressing has effects of moistening the skin, protecting the heart, conditioning the premenstrual syndrome and the like by adding anthocyanin; and the gel dressing can effectively improve human immunity, moisten genital tracts, protect mucous membranes and effectively relieve vaginal dryness and other symptoms by adding carrageenan.
Owner:重庆宫妙生物科技有限公司
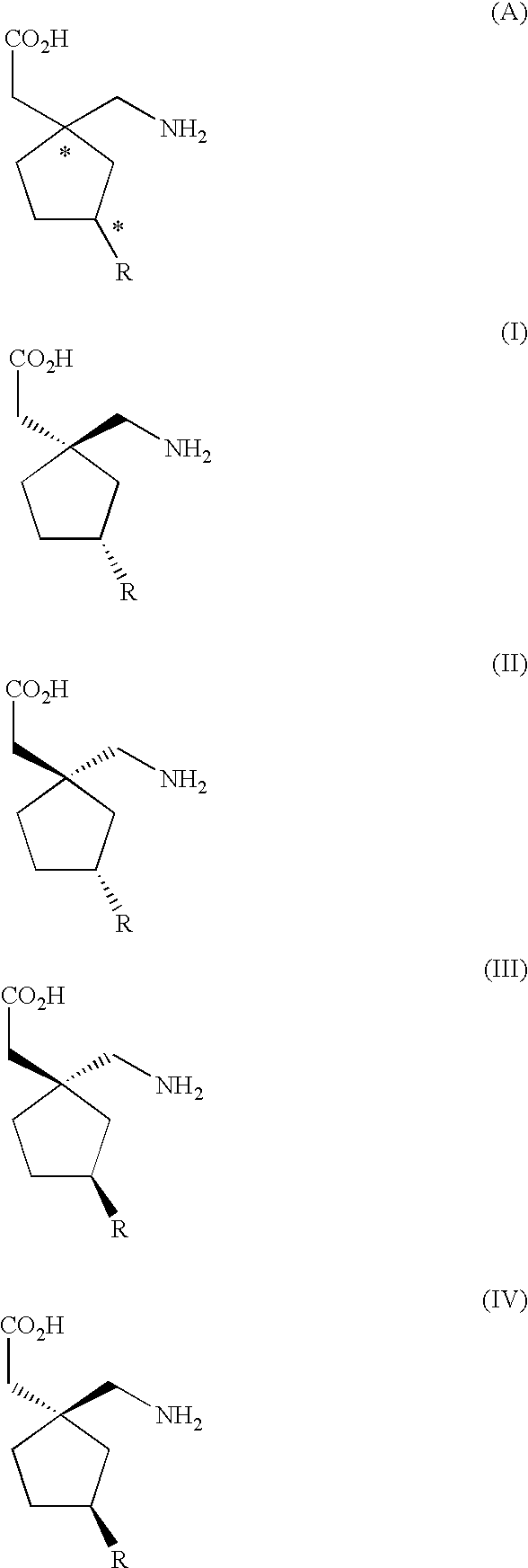
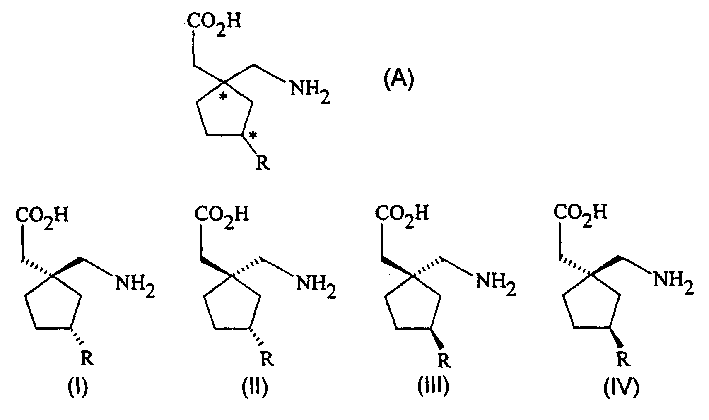
Stereoselective synthesis of cyclic amino acids
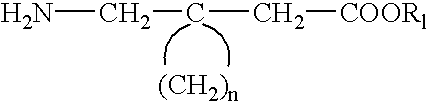
The instant invention is a route to stereospecific 3-substituted 5-membered ring isomers of Formula (A). The final products are useful as agents in the treatment of epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, neuropathological disorders, gastrointestinal disorders such as irritable bowel syndrome (IBS), inflammation especially arthritis, sleep disorders, premenstrual syndrome, and hot flashes. The invention provides novel routes to synthesize stereoselectively analogs of gabapentin (Neurontin3) of Formulas (I), (II), (III) and (IV) wherein R is C1-C10 alkyl or C3-C10 cycloalkyl and pharmaceutically acceptable salts thereof.
Owner:WARNER-LAMBERT CO
Spiroindoline derivatives as gonadotropin-releasing hormone receptor antagonists
InactiveCN104169287AOrganic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorDisease
Owner:BAYER IP GMBH
Edible health borage oil microcapsule for women and preparation method of borage oil microcapsule
ActiveCN104382021AIncrease elasticityRestore lusterSugar food ingredientsLipidic food ingredientsBiotechnologySodium Caseinate
The invention discloses an edible health borage oil microcapsule for women and a preparation method of the borage oil microcapsule. The capsule mainly comprises the following main raw materials: borage oil, corn syrup and sodium caseinate. The preparation method of the borage oil microcapsule comprises the following steps: homogenizing the raw materials under high pressure, spray drying the raw materials and preparing the raw materials into powder, sieving and packing to obtain borage oil microcapsule powder. The borage oil microcapsule prepared by the method is and is safe and non-toxic, and raw materials are easily available; the preparation method is simple in process, low in cost and suitable for large-scale production. The borage oil microcapsule can be widely used in solid beverage powder such as milk tea, milk powder and the like so as to provide a health food for women with the effects of relieving premenstrual syndrome and climacteric syndrome, improving skin elasticity and restoring the skin gloss.
Owner:NANCHANG UNIV
Nutrition food product for improving females climacteric health
The invention relates to nutritional food with effect of improving health of female in climacteric stage, which comprises (wt%) calcium carbonate 6.25-25.00, soybean protein isolate 6.25-12.50, spirulina 12.50-25.00, soybean isoflavones 1.25-3.12, Angelica sinensis extract 0.62-3.75, rhizoma polygonati extract 0.62-2.50, acanthopanax senticosus extract 5.00-11.25, schisandra chinensis fruit extract 1.25-3.75, vitamin C 0.75-1.25, vitamin E 0.25-0.62, ferric sodium ethylenediamine tetracetate 0.25-1.25, microcrystalline cellulose 64.81-9.61, and polyethylene glycol 0.20-0.40. The inventive food has the effects of improving premenstrual syndrome and female physiological function, retaining normal secretion of hormone, relieving climacteric symptoms, promoting metabolic function of human body and organs, relieving osteopenia, and delaying aging.
Owner:北京东方兴企食品工业技术有限公司
2-halo-5-alkynyl-pyridyl nicotinic ligands
Disclosed are heterocyclic compounds that are ligands for nicotinic acetylcholine receptors. The compounds are useful for treating a mammal suffering from any one of a range of therapeutic indications, including Alzheimer's disease, Parkinson's disease, dyskinesias, Tourette syndrome, schizophrenia, attention deficit disorder, anxiety, pain, depression, obsessive compulsive disorder, chemical substance abuse, alcoholism, memory deficit, pseudodementia, Ganser's syndrome, migraine pain, bulimia, obesity, premenstrual syndrome or late luteal phase syndrome, tobacco abuse, post-traumatic syndrome, social phobia, chronic fatigue syndrome, premature ejaculation, erectile difficulty, anorexia nervosa, disorders of sleep, autism, mutism, trichotillomania, and hypothermia.
Owner:GEORGETOWN UNIV
Traditional Chinese medicine composition and making method thereof
PendingCN111228470AIncrease elasticityReduce the burden onOrganic active ingredientsNervous disorderBiotechnologyPimple
The invention discloses a traditional Chinese medicine composition and a making method thereof. The traditional Chinese medicine composition consists of the following components in parts by weight: 18-22 parts of a kudzu-vine root extract, 1-6 parts of a cortex eucommiae male flower extract, 12-20 parts of a sunflower pollen extract, 18-25 parts of a herba cistancheextract, 5-13 parts of a paeoniaostii flower extract, 0.1-0.2 part of tripotassium glycyrrhinate and 1-15 parts of nattokinase. Effective components are extracted from new resources and raw materials as both medicines and foods, symptoms such as hypertension and thrombi can be substantially treated, thrombi can be effectively eliminated, blood vessel elasticity can be improved, heart burden can be alleviated, and hearts can beeffectively protected. The traditional Chinese medicine composition has unique treatment effects on climacteric syndromes, endocrine dyscrasia, menstruation ache, amenorrhea metrorrhagia and metrostaxis, pimples, acnes, female premenstrual syndromes, upset, restlessness, head disturbance caused by deficiency fire, nervousness and chest oppression, dizziness and headache, color stains, and the like.
Owner:刘神剑
Compound and composition for use in treatment of premenstrual syndrome and/or premenstrual dysphoric disorder
PendingCN113840614ANervous disorderPharmaceutical delivery mechanismPhysiologyPremenstrual dysphoric disorder
The present invention relates to a compound and a composition for use in the treatment of premenstrual syndrome and / or premenstrual dysphoric disorder.
Owner:SYNAPHARM IND SYNTHESIS
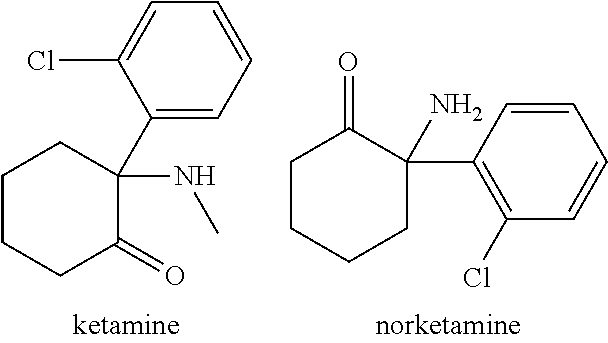
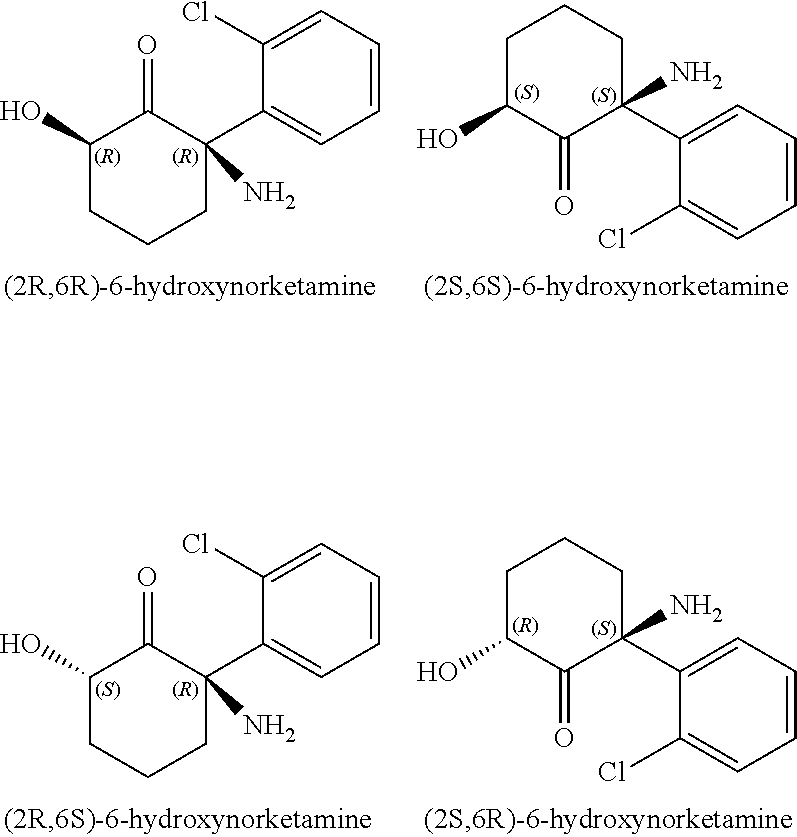
Ketamine for the treatment of menstrually related symptoms
PendingUS20210113493A1Reduce the possibilityRapid introductionNervous disorderOrganic chemistryDiseaseDuring menopause
The present invention features methods, compositions, and kits for the treatment of all forms of emotional and painful symptoms related to the menstrual cycle, including symptoms characteristic of menstrual cycle-related disorders, such as premenstrual dysphoric disorder (PMDD), premenstrual syndrome (PMS), menopause, perimenopause, and sub-diagnostic emotional, psychological, cognitive, spiritual, and / or physical symptoms of the menstrual cycle.
Owner:WOLFSON PHILIP E
Antidepressant azaheterocyclylmethyl derivatives of 7,8-dihydro-6H-5-oxa-1-aza-phenanthrene
Compounds of the formula useful for the treatment of such as depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as premenstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder (including trichotillomania), social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa, bulimia nervosa, vasomotor flushing, cocaine and alcohol addition, sexual dysfunction (including premature ejaculation), and related illnesses.
Owner:WYETH LLC
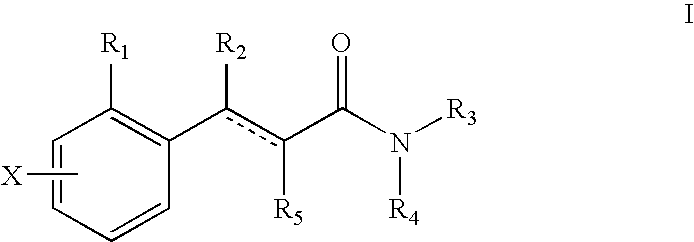
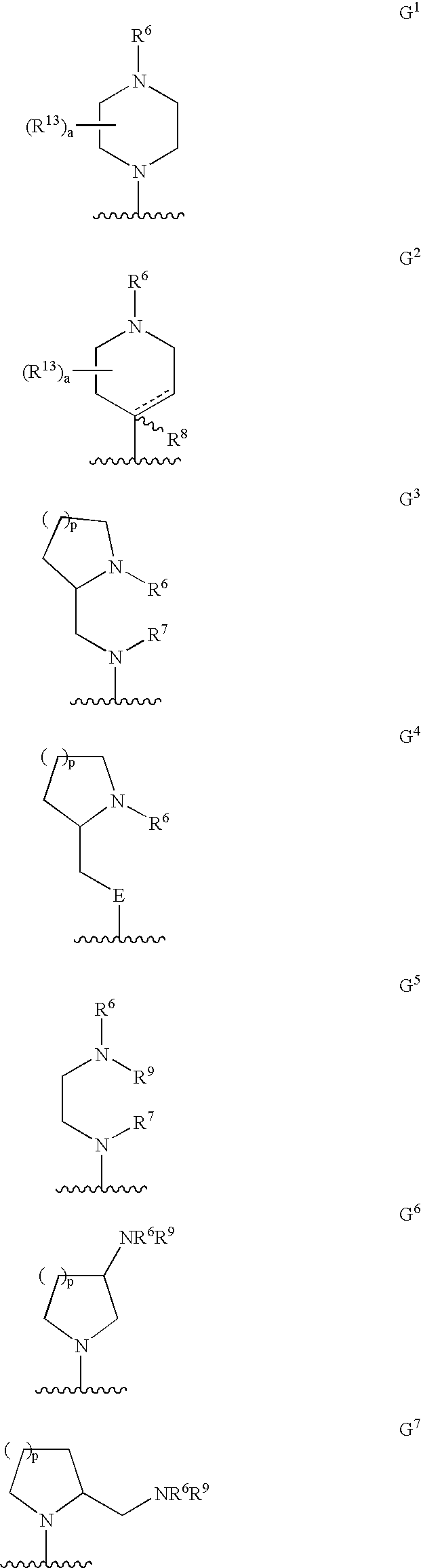
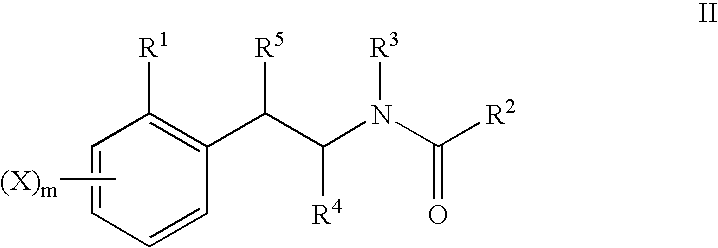
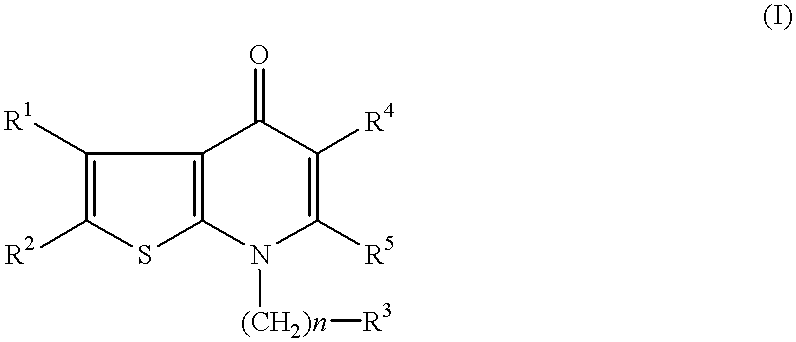
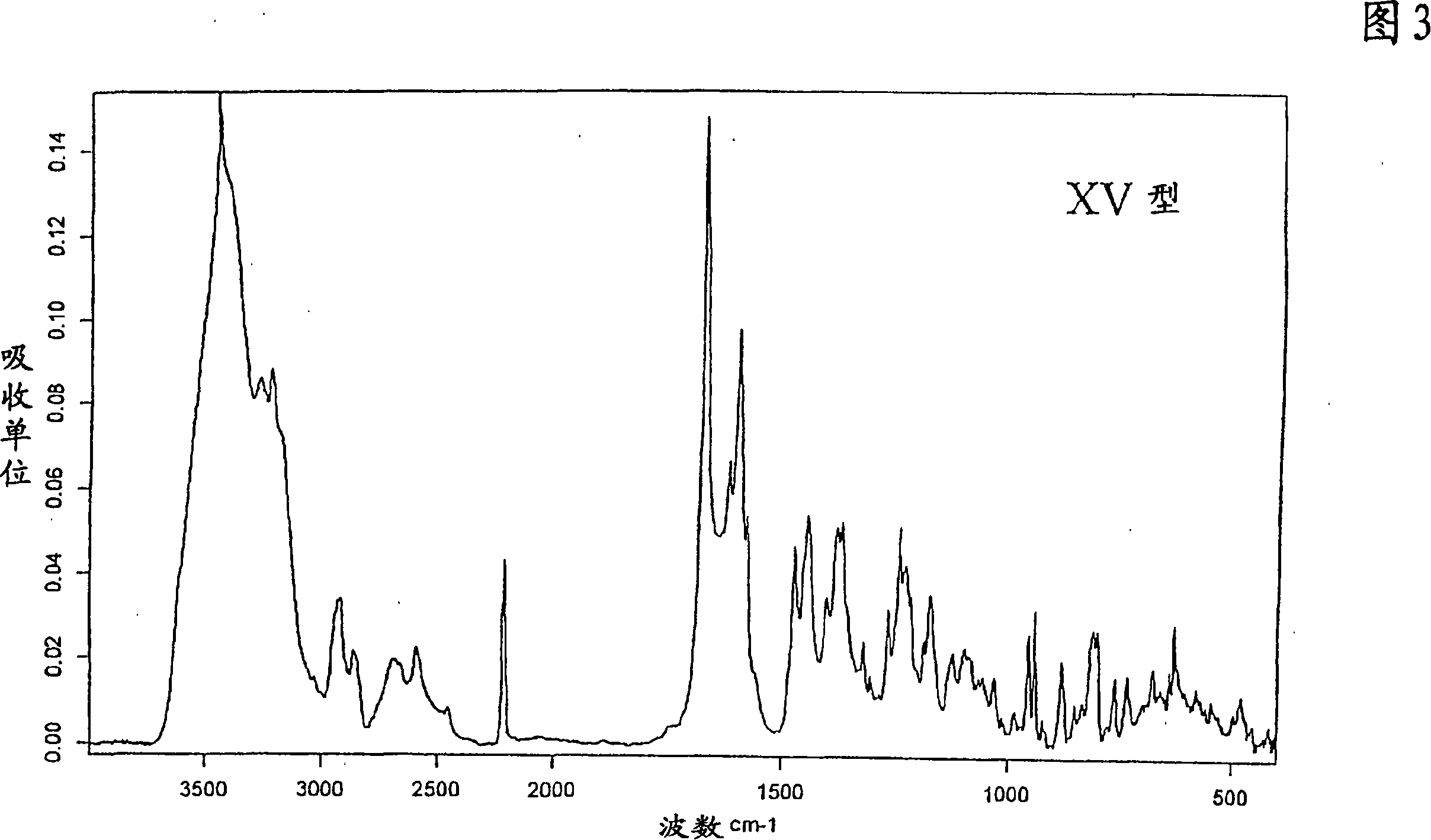
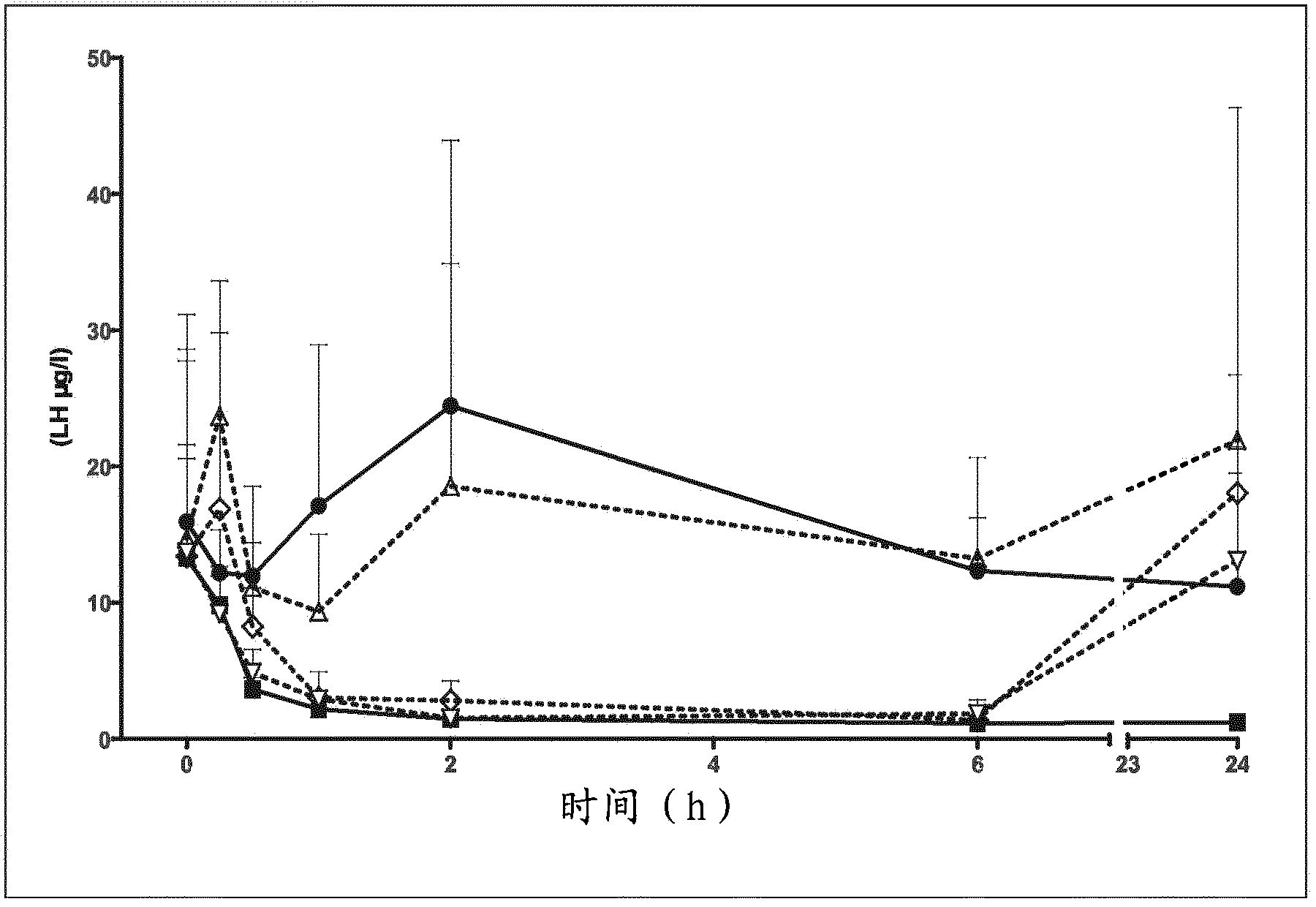
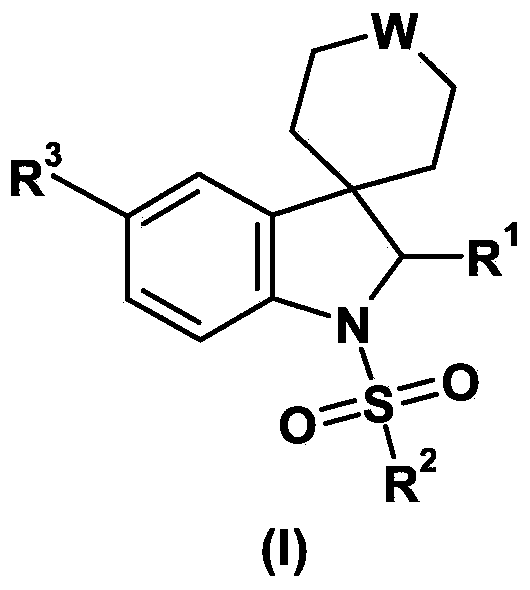
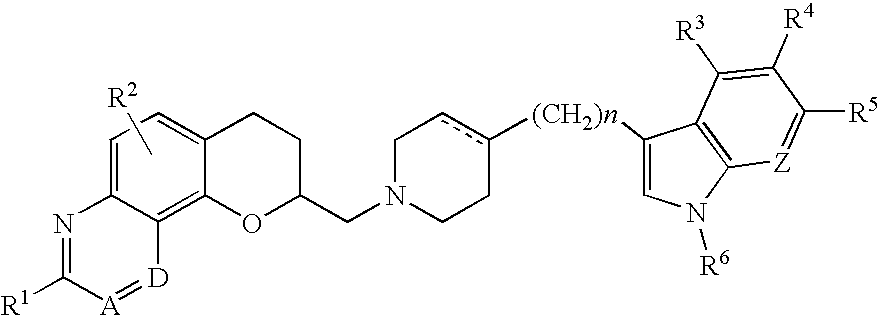
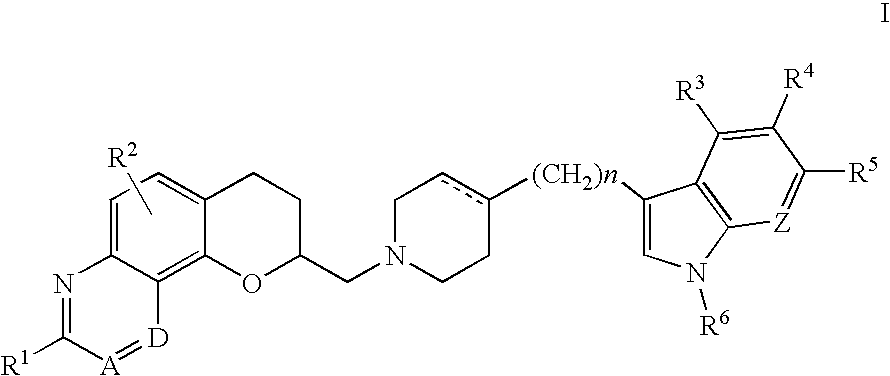
Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same
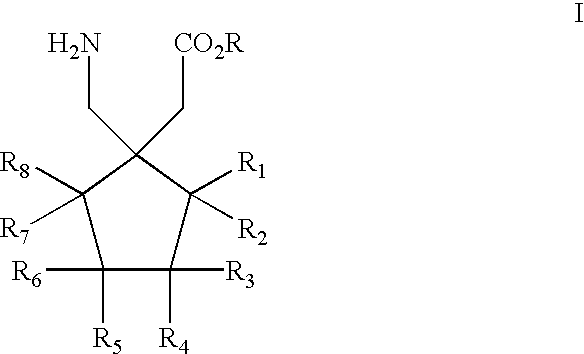
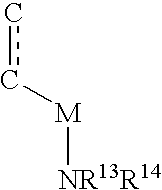
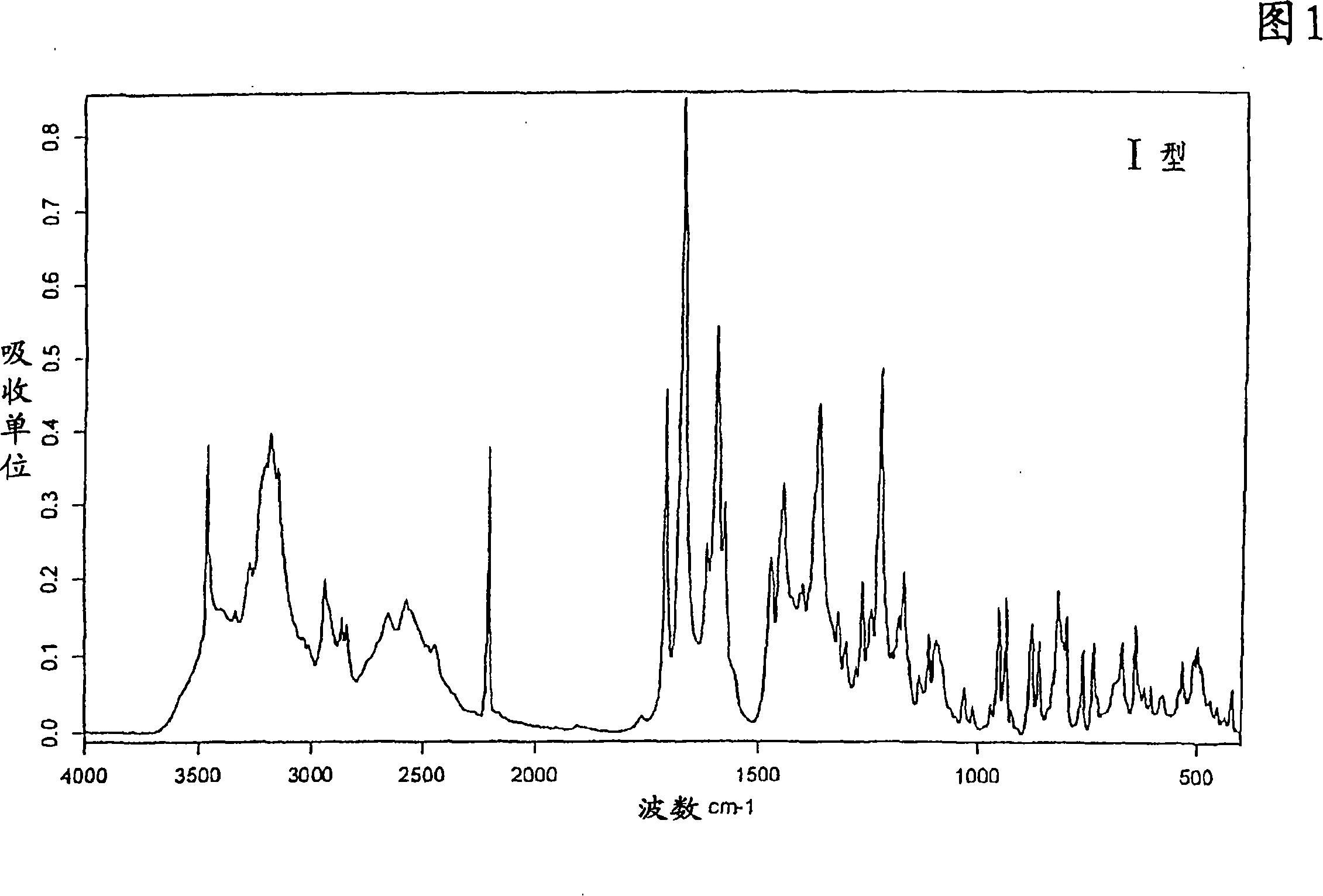
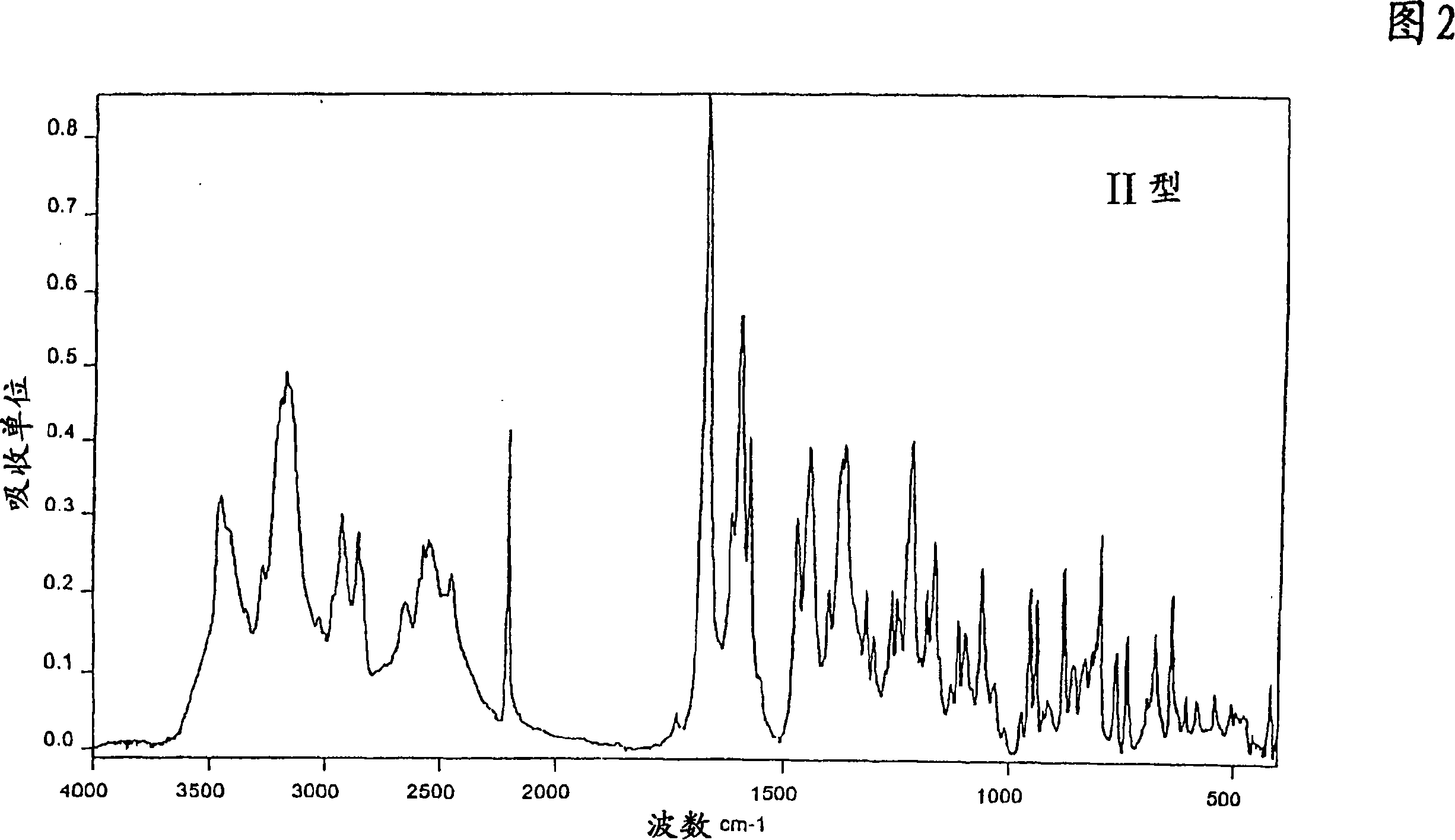
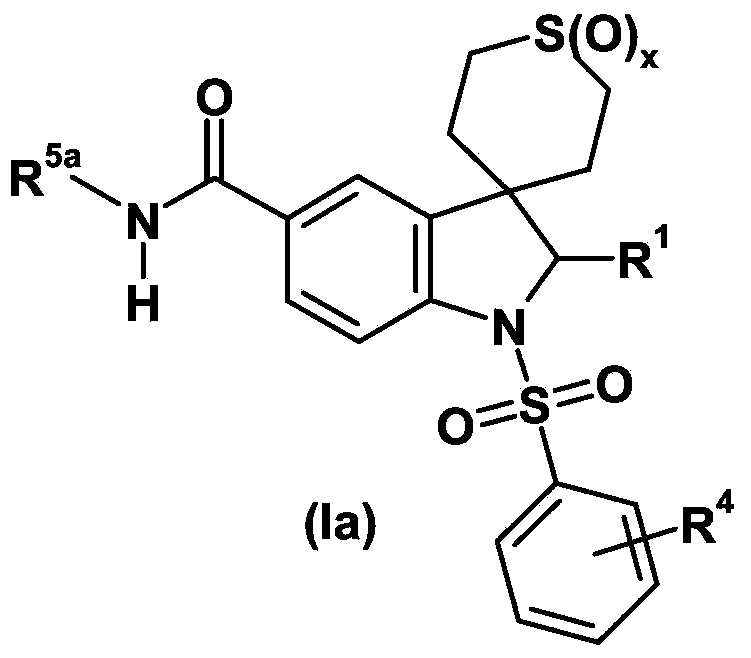
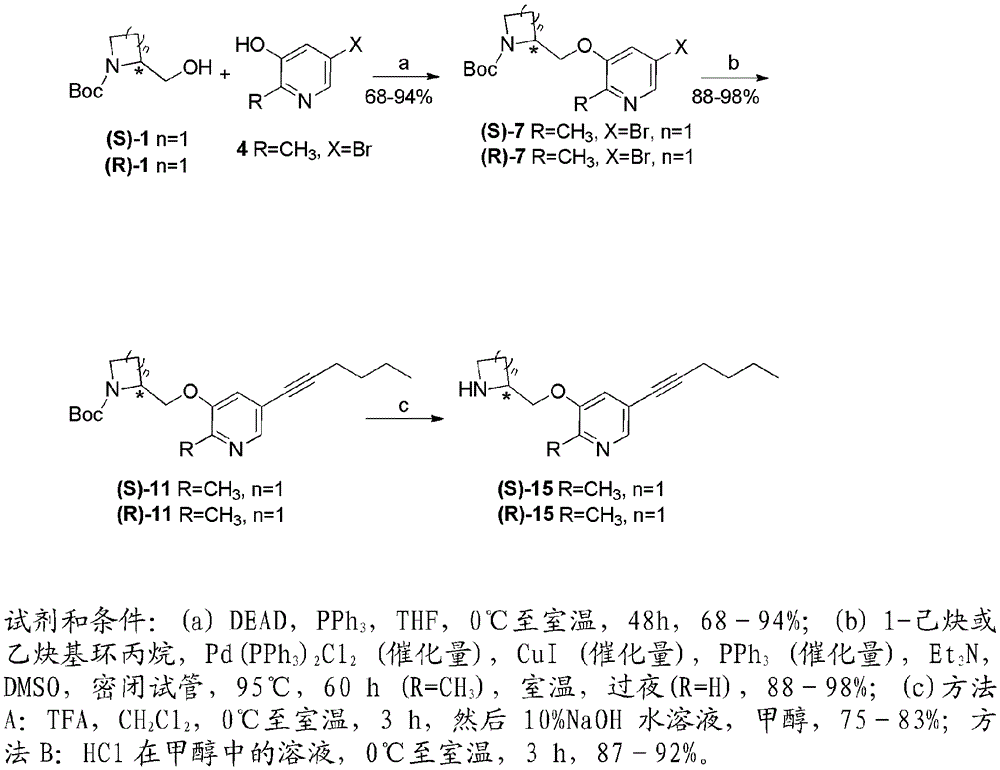
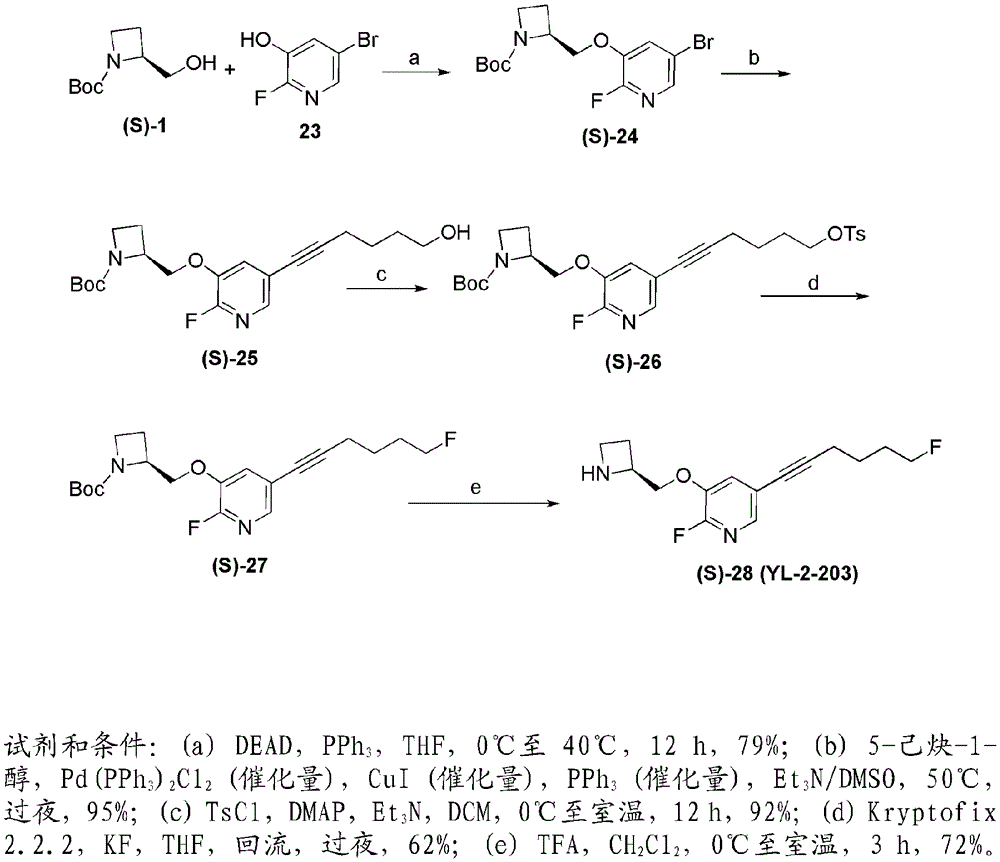
The invention relates to a deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and a pharmaceutical composition containing the compound. Specifically, the invention discloses the deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound shown as a formula (I) and a pharmaceutical composition containing the compound, or a crystal form, a pharmaceutically acceptable salt, a hydrate or a solvate thereof. The compound provided by the invention can be used for treating and / or preventing gonadotropin releasing hormone (GnRH) dependent related diseases, such as hysteromyoma, endometriosis, hysterofibroma, amenorrhea, premenstrual syndrome and the like.
Owner:SUZHOU ZELGEN BIOPHARML +1
Composition for ameliorating premenstrual syndrome symptoms, including chrysanthemum zawadskii extract
PendingUS20220280583A1Inhibition of secretionIncrease secretionSexual disorderEndocrine system disorderPhysiologyChrysanthemum zawadskii
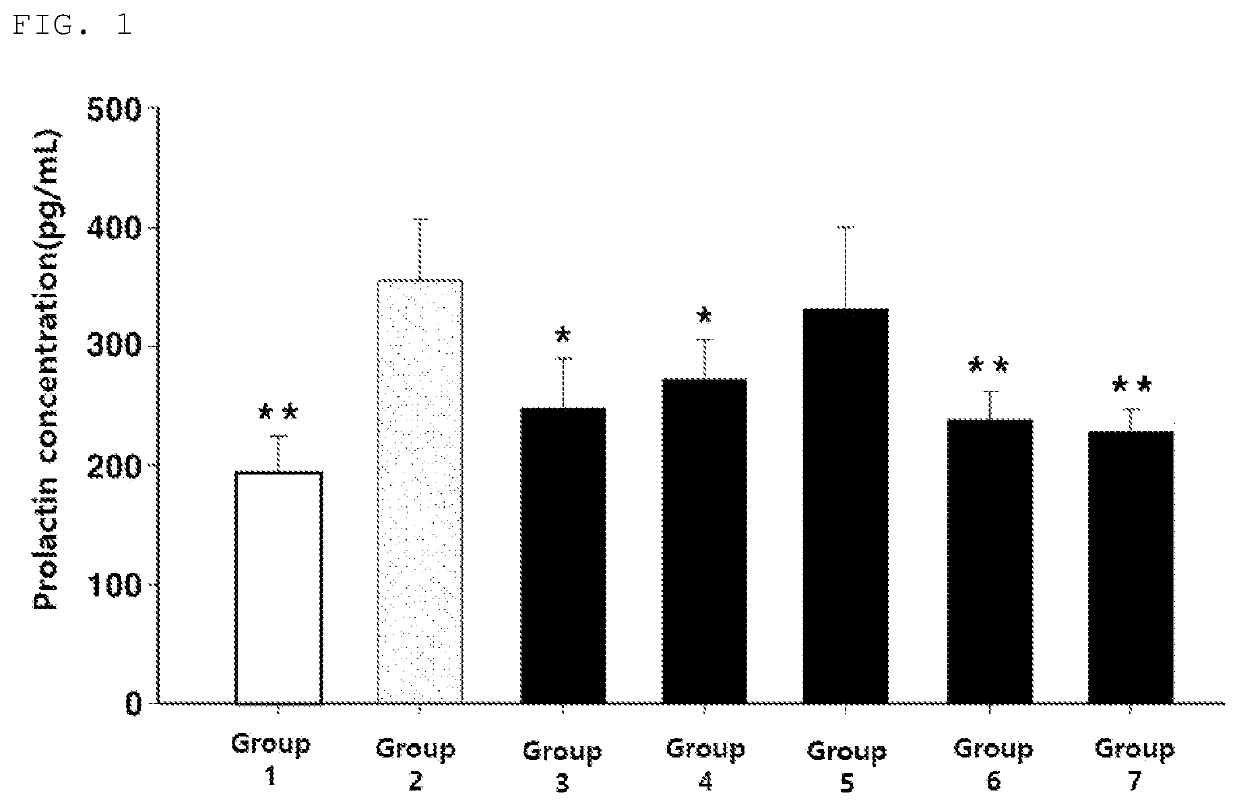
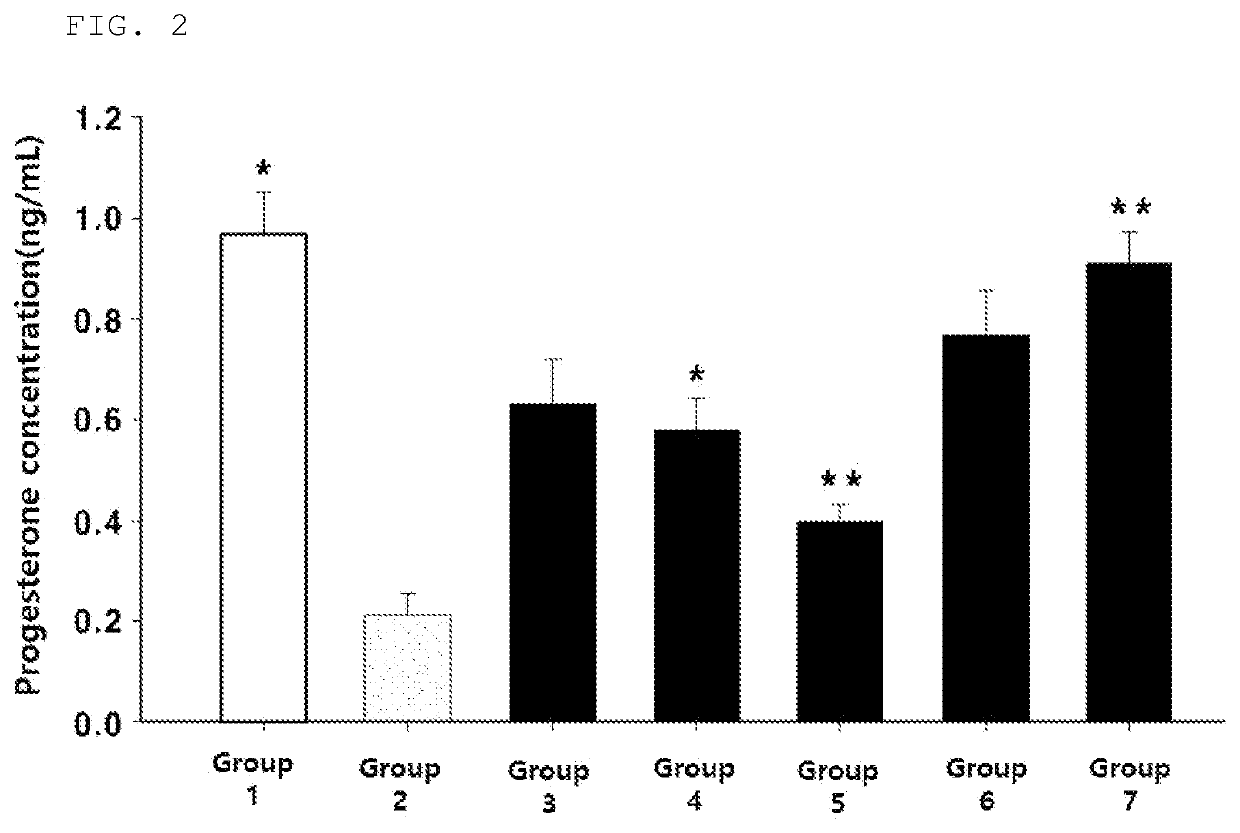
The present invention relates to a composition which is for preventing and ameliorating or treating premenstrual syndrome symptoms, and includes: (a) a Chrysanthemum zawadskii extract; (b) a mixture of the Chrysanthemum zawadskii extract and a malt extract; or (c) a mixture of the Chrysanthemum zawadskii extract, the malt extract, and an aloe extract. Specifically, the present compositions effectively inhibit the secretion of prolactin from pituitary cells, which is a phenomenon that appears during premenstrual syndrome, thereby increasing the secretion of progesterone, which is reduced during the luteal phase of women, and can thus be advantageously used as a composition for preventing and ameliorating or treating women's premenstrual syndrome.
Owner:GENENCELL INC
A traditional Chinese medicine composition for preventing and treating menstrual syndrome and its preparation method
ActiveCN108159261BHas a sedative effectImprove menstrual syndromeOrganic active ingredientsAntipyreticSucroseCALCIUM LACTOBIONATE
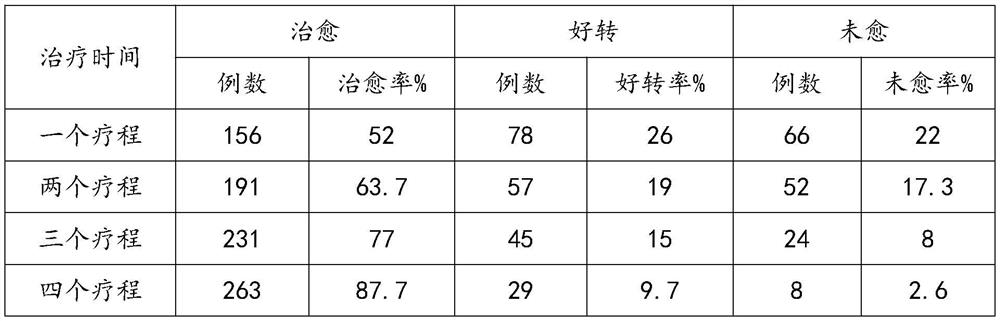
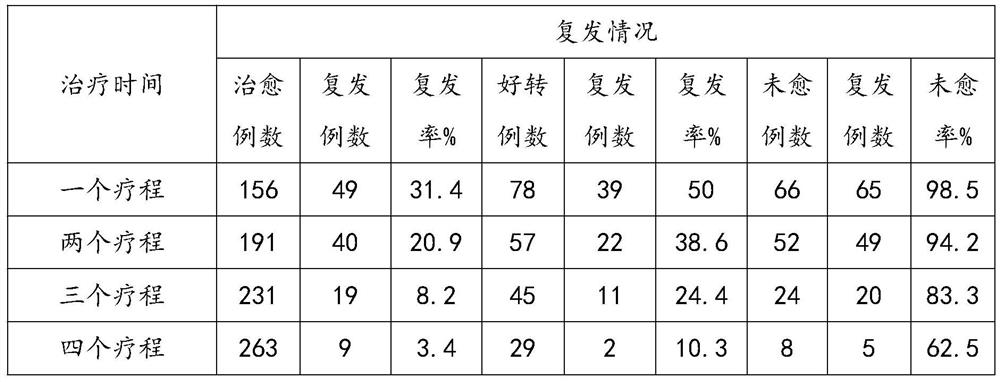
The invention discloses a traditional Chinese medicine composition for preventing and treating premenstrual syndromes and a preparation method of the traditional Chinese medicine composition, relatesto the technical field of traditional Chinese medicines and aims to solve the technical problems that a conventional medicine for treating premenstrual syndromes is not remarkable in treatment effect,and the like. The traditional Chinese medicine composition comprises the following active components in parts by mass: 20 parts of theanine, 10 parts of gelatine powder, 3-5 parts of polygonatum sibiricum powder, 9-11 parts of lycium barbarum powder, 0.8-1.2 parts of radix angelicae powder and 0.8-1.2 parts of lotus leaf powder. The preparation method comprises the following steps: mixing the theanine, the gelatine powder, the polygonatum sibiricum powder, the lycium barbarum powder, the radix angelicae powder and the lotus leaf powder; performing dry mixing on sucralose, microcrystalline cellulose, calcium carbonate and calcium lactate; uniformly spraying 70% ethanol, and stirring so as to obtain wet granules; performing drying treatment on the wet granules; performing finishing treatment; mixing with magnesium stearate; inspecting the granules, tableting, screening, coating with membranes, and discharging tablets. The traditional Chinese medicine composition is capable of effectively treating and relieving premenstrual syndromes and is low in cost, high in treatment rate and not liable in disease reoccurrence.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
Alpha 4 beta 2 delta gaba-a receptors as a strategy for pms and alcoholism
InactiveUS20070081943A1Reduce sensitivityHigh sensitivityCompounds screening/testingIn-vivo radioactive preparationsReceptorPharmaceutical drug
The present invention is directed to a screening mechanism for identifying members of the general population at increased risk for alcoholism and premenstrual syndrome. The screening mechanisms may be used to measure the expression of the α4β2δ GABAA receptors, in order to identify members of the general population as having an increased sensitivity to lower concentrations alcohol coupled with a decrease sensitivity to higher concentrations of alcohol, a scenario frequently found in patients suffering from alcoholism and premenstrual anxiety. Methods of screening for drugs which decrease expression of the α4β2δ subunit of GABAA are also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method for the stereoselective synthesis of cyclic amino acids
InactiveUS6864390B2High degree of stereochemical purityOrganic compound preparationOrganic chemistry methodsGabapentinPanic
The instant invention is a route to stereospecific 3-substituted 5-membered ring isomers of Formula (A). The final products are useful as agents in the treatment of epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, neuropathological disorders, gastrointestinal disorders such as irritable bowel syndrome (IBS), inflammation especially arthritis, sleep disorders, premenstrual syndrome, and hot flashes. The invention provides novel routes to synthesize steroselectively analogs of gabapentin (Neurontin®) of Formulas (I), (II), (III) and (IV) wherein R is C1-C10 alkyl or C3-C10 cycloalkyl and pharmaceutically acceptable salts thereof.
Owner:WARNER-LAMBERT CO
Progesterone ethosome, and preparation method and application thereof
ActiveCN102397255BImprove stabilityReduced stabilityOrganic active ingredientsSexual disorderCholesterolActive agent
The invention provides a progesterone ethosome. Progesterone is encapsulated in an ethosome. The progesterone ethosome comprises the following components in percentage by weight: 0.1 to 1 percent of progesterone, 1 to 8 percent of lipid materials, 0 to 0.6 percent of cholesterol, 20 to 50 percent of short-chain alcohols, 0 to 3 percent of nonionic surfactant and the balance of water. The preparation method comprises the following steps of: mixing and dissolving the progesterone, the lipid materials, the cholesterol and the short-chain alcohols to prepare an alcohol phase; with stirring, adding the alcohol phase into a nonionic surfactant-containing aqueous phase to prepare primary emulsion under the condition of stirring; homogenizing the primary emulsion under a high pressure to obtain asuspension; stirring the ethosome suspension for 15 to 30 minutes to perform emulsification for the second time; and curing the suspension by cooling the suspension at 0 to 4 DEG C to obtain the progesterone ethosome. Because the high-pressure homogenization method is adopted to prepare the progesterone ethosome, the progesterone ethosome is low in stimulation to skin and high in transdermal delivery ability, and metal ion pollution which is easy to cause by the probe ultrasound method of the traditional homogenization method is avoided. Therefore, the progesterone ethosome is suitable for industrial production. The progesterone ethosome can be prepared into a transdermal drug delivery system, a mucosal drug delivery system, and topical dosage forms, such as a paster, a gel and the like. The progesterone ethosome is mainly applied to hormone replacement therapy, secondary amenorrhea, functional aplastic bleeding, premenstrual syndrome and the like clinically.
Owner:GUANGDONG PHARMA UNIV
Agent for mitigating hot flash, cosmetic product, and method of using cosmetic product
InactiveUS20210393509A1Highly effectively mitigating hot flashesCosmetic preparationsToilet preparationsHot flashes/flushesBULK ACTIVE INGREDIENT
Disclosed is a hot flash-mitigating agent that is highly effective in mitigating hot flashes associated with premenstrual syndrome. The hot flash-mitigating agent contains mallow extract as an active ingredient.
Owner:BLOOM CLASSIC CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same](https://images-eureka.patsnap.com/patent_img/5a084b77-ab26-43c0-9845-f56bb3a4774f/HDA0002494499990000011.png)
![Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same](https://images-eureka.patsnap.com/patent_img/5a084b77-ab26-43c0-9845-f56bb3a4774f/HDA0002494499990000012.png)
![Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same Deuterated tetrahydrothieno [3, 4-d] pyrimidinedione compound and pharmaceutical composition containing same](https://images-eureka.patsnap.com/patent_img/5a084b77-ab26-43c0-9845-f56bb3a4774f/FDA0002494499970000011.png)