Fusion protein of thrombopoietin mimetic peptide (TMP) diad and human serum albumin (HSA), and preparation method and application thereof
A technology of thrombopoietin and human serum albumin, applied in the field of biotechnology and genetic engineering pharmaceuticals, can solve the problems of short biological half-life, unsatisfactory application effect, small molecular weight, etc., and achieve the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the total gene synthesis of human serum albumin HSA
[0068] 1. First, optimize the gene sequence encoding HSA (including signal peptide and propeptide consisting of 24 amino acid residues) according to the preferred codons of Pichia pastoris. The optimized full-length gene sequence of HSA is shown in SEQ ID NO: 10 The 1-72 bp at the 5' end is the base sequence encoding HSA signal peptide and propeptide. The signal peptide and propeptide are automatically excised during expression and secretion in yeast cells.
[0069] 2. Entrust Shanghai Sangon Bioengineering Company to synthesize the optimized full-length gene of HSA and load it into pUC57 plasmid (the pUC57 plasmid vector is provided by Shanghai Sangon Biotechnology Company) to obtain pUC57-HSA.
Embodiment 2
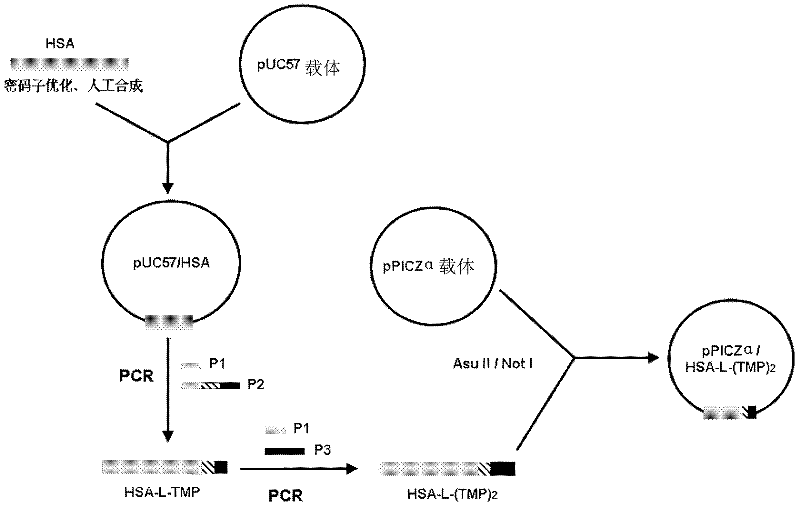
[0070] Example 2: pPICZα / HSA-L-(TMP) 2 Construction of recombinant expression vector
[0071] 1. Code HSA-L-(TMP) 2 Obtaining of cDNA fragments
[0072] (1) Design and synthesize PCR primers:
[0073] P1 (SEQ ID NO: 7): 5'-agc ttc gaa acg_atg aag tgg gta acc ttt att tcc ctt c 3′
[0074] P2 (SEQ ID NO: 8): 5'-ggc tct agc agc caa cca ttg tct caa agt tgg acc ctc aat acc aga tcc acc gcc tcc aga tcc acc tcc gcc gga tcc taa gcc taa ggc agc ttg ac-3'
[0075] P3 (SEQ ID NO:9): 5'-at cgc ggc cgc tta tgc tct agc tgc aag cca ctg tct cag ggt agg tcc ctc gat tgg acc aga tgg ggc tct agc agc caa cca ttg-3′
[0076] Among them, the base underlined in P1 is the recognition site sequence of restriction endonuclease Asu II; the 5' end of P2 is the sequence encoding TMP, the italic part in the middle is the Linker sequence between HSA and TMP, and the 3' end is the HSA gene C-terminal specific recognition sequence; the base underlined in P3 is the restriction endonuclease Not I recog...
Embodiment 3
[0082] Example 3: HSA-L-(TMP) 2 Expression and purification of fusion proteins
[0083] Take the integrated pPICZα / HSA-L-(TMP) frozen at -70℃ 2 The engineered yeast strains were inoculated on the YPDS plate at 30°C for overnight culture and activation, and a single colony with a good appearance was selected and inoculated in the BMGY medium, and cultured at 220rpm on a constant temperature shaker at 30°C for 16-18 hours to OD 600 ≈3~5, then transfer to OD once at 1:10 600 It is about 4, and the bacterial liquid is used as the seed liquid. Then the seed solution was transplanted into a 15L fermenter (Bio Company, Switzerland) equipped with 7L basic salt medium for high-density culture and fermentation. Each liter of basal medium contains 50g of glycerol, 12ml of concentrated phosphoric acid, 2.6g of KOH, CaSO 4 2H 2 O 0.6g, K 2 SO 4 9.5g, MgSO 4 ·7H 2 O 7.8g, biotin 0.32mg, YTB solution (containing 65g / L of FeSO 4 ·7H 2 O, 6g / L CuSO 4 ·5H 2 O, 20g / L ZnSO 4 ·7H 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com