Preparation method of ultraviolet-light-resistant thiophene-structure-containing polybenzoxazole fibre
A polybenzoxazole, UV-resistant technology, applied in the direction of chemical characteristics of fibers, one-component synthetic polymer rayon, textiles and papermaking, etc. and other problems, to achieve the effects of excellent UV resistance, excellent uniformity and serious equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
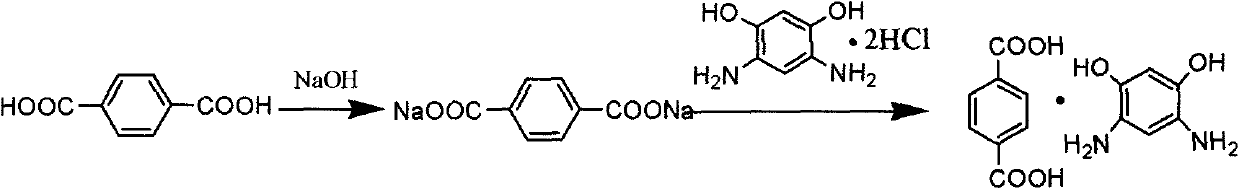
[0033] Preparation of 4,6-diaminoresorcinol / terephthalic acid compound salt
[0034]In a 125mL four-neck flask equipped with stirring, condenser and thermometer, add 10.66g (0.05mol) 4,6-diaminoresorcinol dihydrochloride and 100mL deionized water under the protection of nitrogen inert gas, and Dissolve 9.30g (0.05mol) of terephthalic acid in 200g of aqueous sodium hydroxide (the concentration of sodium hydroxide is 2%) to prepare an aqueous solution of disodium terephthalic acid, and add the solution dropwise to the above-mentioned solution at 80°C. In 4,6-diaminoresorcinol dihydrochloride aqueous solution, react at 80°C for 10 minutes, filter, wash, filter again and vacuum dry to obtain 4,6-diaminoresorcinol / terephthalic acid compound salt.
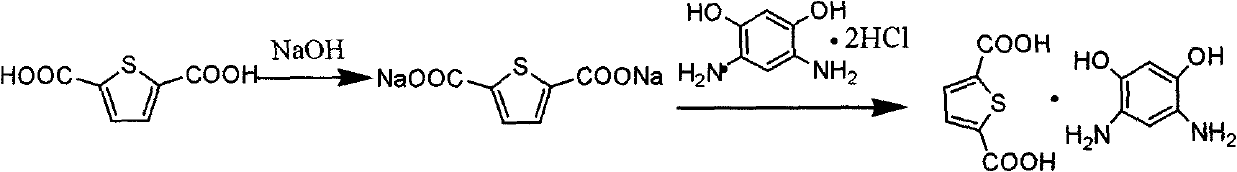
[0035] Preparation of 4,6-diaminoresorcinol / thiophenedicarboxylic acid compound salt
[0036] In a 125mL four-neck flask equipped with stirring, condenser and thermometer, add 10.66g (0.05mol) 4,6-diaminoresorcinol dihydrochloride ...
Embodiment 2
[0041] The preparation process of 4,6-diaminoresorcinol / terephthalic acid compound salt and 4,6-diaminoresorcinol / thiophenedicarboxylic acid compound salt is the same as in Example 1.
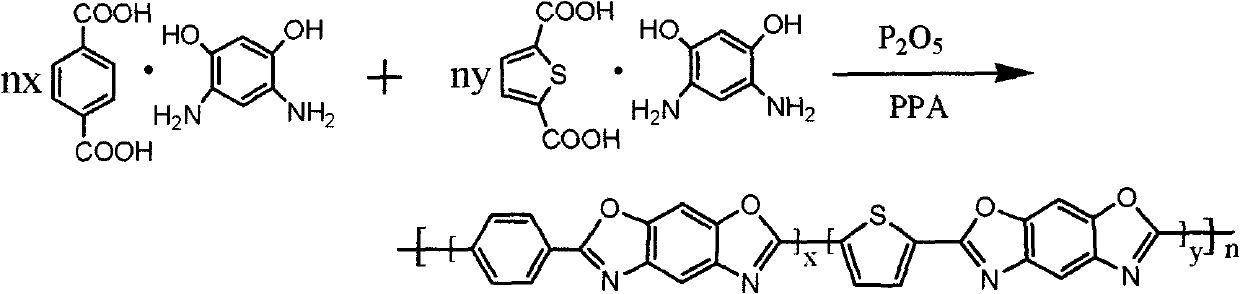
[0042] In a 125mL four-neck flask equipped with stirring, condenser tube, drying tube and thermometer, add 12.4g (0.04mol) 4,6-diaminoresorcinol / terephthalic acid compound salt, 3.12g (0.01mol) 4,6-diaminoresorcinol / thiophenedicarboxylic acid compound salt, 10.5g phosphorus pentoxide (content in P 2 o 5 Calculated as 84%), 119.1g polyphosphoric acid, stirred and heated to 120°C for 6h, and slowly raised the temperature to 30°C for 6h, 150°C for 12h, 180°C for 2h, and 200°C for 1h. The liquid is transferred to the spinning equipment, and the dry-jet wet spinning process is used to make fibers. The obtained fibers are further removed with 5% sodium hydroxide aqueous solution and water to remove residual phosphoric acid, and then dried in a vacuum drying oven to obtain a golden yellow thiophene-c...
Embodiment 3
[0045] The preparation process of 4,6-diaminoresorcinol / terephthalic acid compound salt and 4,6-diaminoresorcinol / thiophenedicarboxylic acid compound salt is the same as in Example 1.
[0046] In a 125mL four-neck flask equipped with stirring, condenser, drying tube and thermometer, add 14.99g (0.049mol) 4,6-diaminoresorcinol / terephthalic acid compound salt, 0.31g (0.001mol) 4,6-diaminoresorcinol / thiophenedicarboxylic acid compound salt, 12.60g phosphorus pentoxide, 91.36g polyphosphoric acid (content in P 2 o 5 Calculated as 84%), stirred and heated to 120°C for 6h, and slowly raised the temperature to 130°C for 12h, 150°C for 12h, 180°C for 2h, and 200°C for 1h. After the reaction was completed, the reaction solution was transferred to the spinning equipment In the process, dry-jet wet-spinning technology is used to make fibers, and the obtained fibers are further removed with 5% sodium hydroxide aqueous solution and water to remove residual phosphoric acid, and then dried ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com