Preparation method of catalyst in process of producing light olefins by high-activity load type iron-based synthesis gas
A low-carbon olefin, supported technology, which is applied in the field of preparation of high-activity supported iron-based synthesis gas to low-carbon olefin catalyst, can solve the problems of difficult to achieve reactivity, low CO conversion rate, and high catalyst cost, and achieve mechanical The effect of high strength, improved activity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
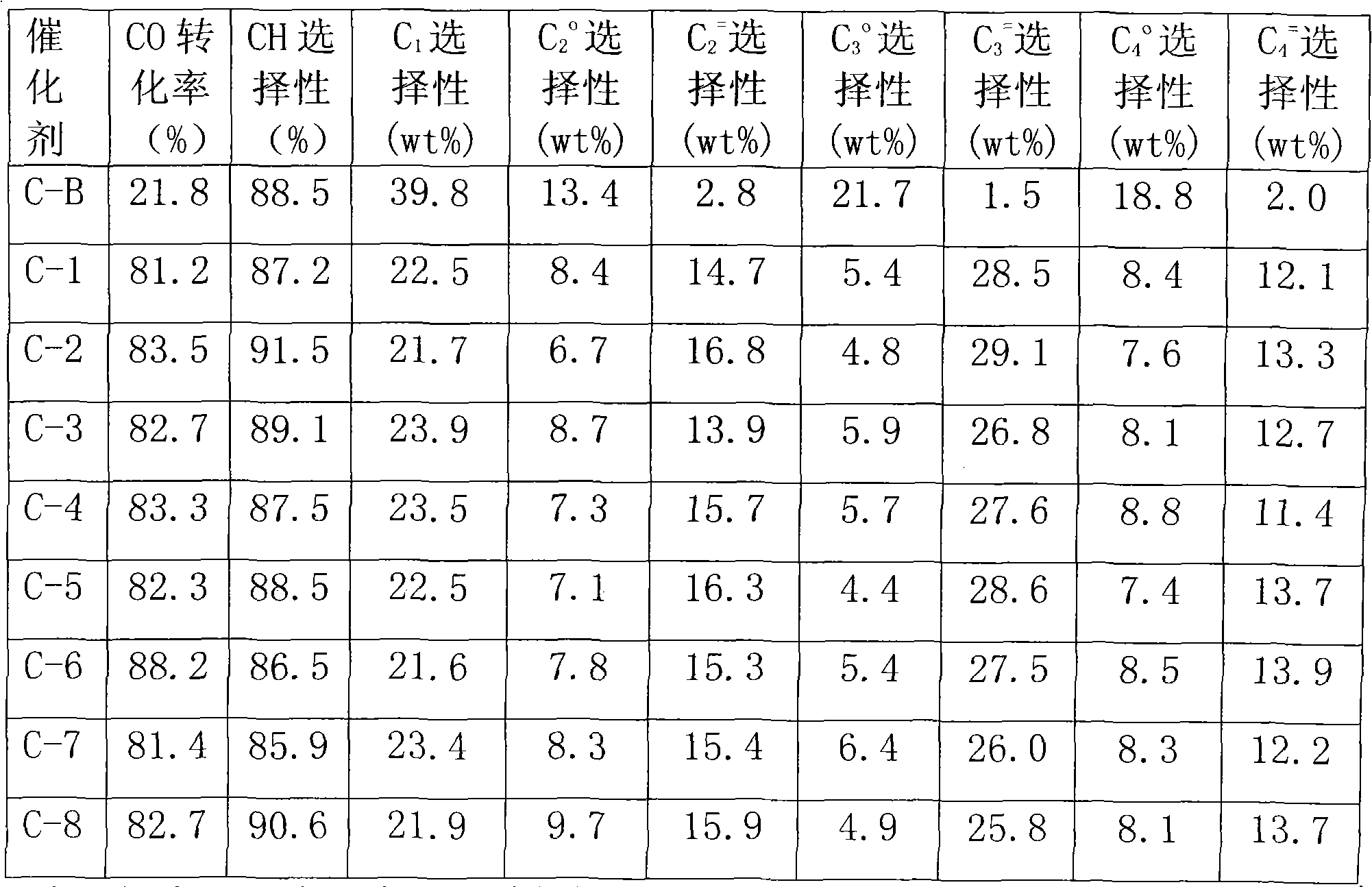
[0018] Weigh commercially available silica gel (pore volume is 1.06ml / g, specific surface area is 386.81m 2 / g, the following examples all use the silica gel) 30g, distilled water is added dropwise to initial moistening, the volume of consumed water is 48ml, 48ml concentration is 5% ammonium carbonate solution, add in silica gel at 50 ℃, process 10 hours. Dry at 60°C for 24 hours, then bake at 280°C for 15 hours. Based on the final catalyst K content of 0.023wt%, weigh 0.0179g of potassium nitrate and add distilled water to 48g, add the above-mentioned modified carrier silica gel for impregnation, dry at 60°C for 24 hours, and bake at 350°C for 10 hours. Based on the final catalyst Fe content of 3wt%, weigh 6.6303g of ferric nitrate and add distilled water to 48g, add to the above-mentioned sample impregnated with potassium, dry at 60°C for 24 hours, and bake at 350°C for 10 hours in vacuum or nitrogen atmosphere. Based on the final catalyst manganese content of 1.06wt%, weig...
example 2
[0021] Weigh 30g of commercially available silica gel, add distilled water dropwise until initial moistening, the volume of consumed water is 48ml, add 48ml of 15% ammonium carbonate solution to the silica gel at 80°C, and stir. Treat for 30 hours and dry at 90°C for 16 hours. According to the final catalyst K content of 0.39wt%, weigh 0.3042g of potassium nitrate, add distilled water to 48g, add the above-mentioned modified carrier silica gel to impregnate, dry at 100°C for 16 hours, and roast in vacuum or nitrogen atmosphere at 550°C for 4 Hour. Based on the final catalyst Fe content of 9wt%, weigh 19.8909g of ferric nitrate, add distilled water to 48g, add the above-mentioned potassium-impregnated sample, dry at 100°C for 16 hours, and bake in vacuum or nitrogen atmosphere at 550°C for 4 hours. Based on the final catalyst manganese content of 3.6wt%, weigh 7.0355g of 50% manganese nitrate solution, add distilled water to 48g, add the above sample impregnated with potassium...
example 3
[0023]Weigh 30g of commercially available silica gel, add distilled water dropwise until initial moistening, the volume of consumed water is 48ml, add 48ml of ammonium carbonate solution with a concentration of 20% to the silica gel at 95°C, stir for 100 hours, and dry at 100°C for 8 hours . Based on the final catalyst K content of 0.8wt%, 0.624g of potassium nitrate was weighed and dissolved in 48ml, added to the modified carrier silica gel for impregnation, dried at 150°C for 8 hours, and roasted at 700°C for 2 hours in vacuum or nitrogen atmosphere. Based on the final catalyst Fe content of 12wt%, 26.5212g of iron nitrate was weighed and dissolved in 48ml, added to the above-mentioned sample impregnated with potassium, dried at 150°C for 8 hours, and roasted in vacuum or nitrogen atmosphere at 700°C for 2 hours. Based on the final catalyst manganese content of 5.44wt%, weigh 10.6315g of 50% manganese nitrate solution, add water to 48g, add the above sample impregnated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com