Application of FBP1 (fructose-1,6-bisphosphatase 1) gene
A gene and tumor diagnosis technology, applied in the field of medicine and biology, can solve the problem of unclear relationship between tumors and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
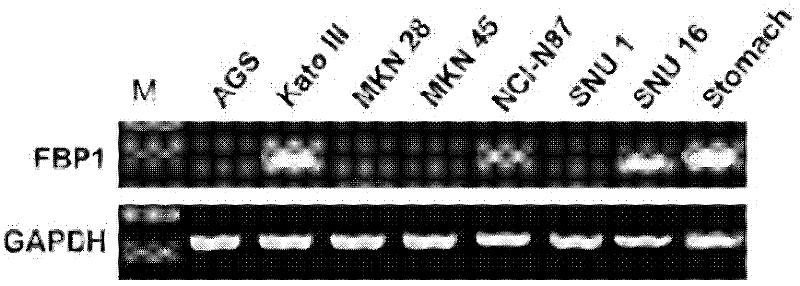
[0027] Example 1 RT-PCR experiment to detect the expression of FBP1 gene in tumor cells
[0028] 1. Cell culture
[0029] All cells were cultured in RPMI-1640 medium containing 100 U / ml penicillin, 100 μg / ml streptomycin and 10% FBS at 37°C in 5% CO 2 cultured in an incubator.
[0030] 2. Total RNA extraction kit
[0031] The total RNA of cells was isolated and extracted with Trizol reagent from Invitrogen Company according to the method provided by the manufacturer. The reagent is produced based on one-step extraction with acidic phenols. The utensils and water used for RNA extraction must be RNase-free to ensure an RNase-free environment in the experiment.
[0032] 3. Extraction of total RNA
[0033] Cultivate the cells to the logarithmic growth phase, drain the culture medium, add 1ml Trizol directly, let stand at room temperature for 5 minutes, and collect the cells into a 1.5ml centrifuge tube. After adding chloroform, centrifuge at 4oC to separate layers; transfe...
Embodiment 2
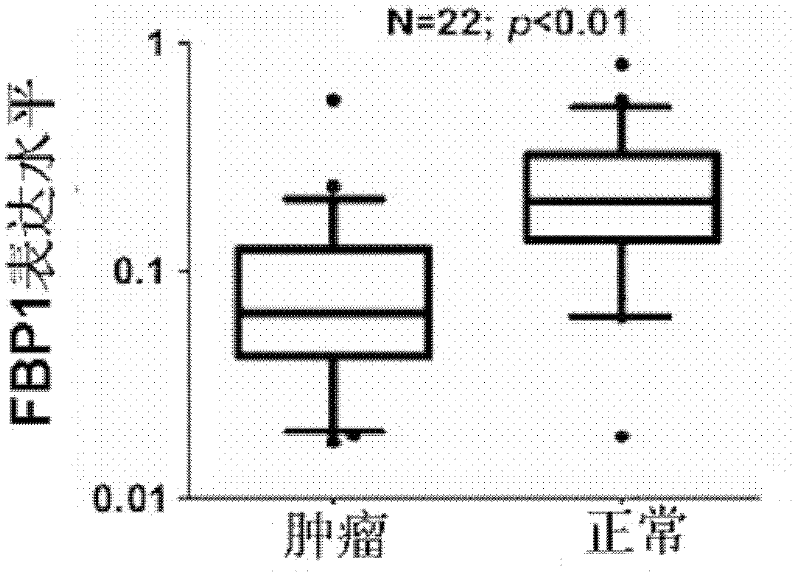
[0047] Example 2 Real-time quantitative PCR experiment to detect the expression of FBP1 gene in tumor tissue
[0048] 1. Tissue Isolation
[0049] All specimens were pathologically confirmed. Once the surgically resected specimens are removed from the body, the tumor lesions and adjacent tissues are quickly excised and stored in liquid nitrogen.
[0050] 2. Total RNA extraction
[0051] Dry-bake the homogenizer at 200oC for 4 hours to remove RNase and cool down; take the tissue out of the liquid nitrogen quickly and grind it into powder; use a spatula to put the tissue into the homogenizer pre-added with TRIzol reagent, and homogenize A few minutes; transfer the homogenized liquid into an RNase-free centrifuge tube, add chloroform, and centrifuge at 4oC to separate layers; transfer the upper aqueous phase to an RNase-free centrifuge tube, add isopropanol, and centrifuge at 4oC Precipitate RNA; wash the pellet twice with 75% ethanol; air-dry and dissolve the pellet in RNas...
Embodiment 3
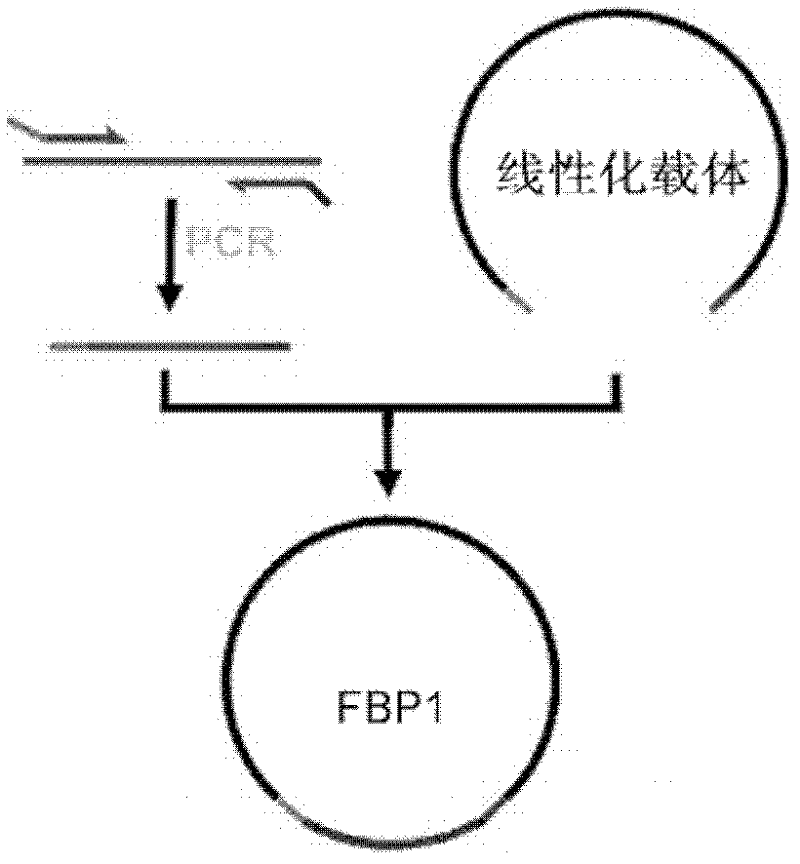
[0057] Cloning of embodiment 3 FBP1 cDNA and construction of expression vector
[0058] 1. Synthesis of clone-specific cDNA
[0059] The reverse transcription reaction (RT) used the SuperScript III First Synthesis System for RT-PCR kit from Invitrogen, USA, and 2 micrograms of total RNA was used in each reaction. The total reaction volume is 20 microliters: 2 microliters of 10×RT buffer, 5 microliters of total RNA, 1 microliter of OligodT20 primer, 1 microliter of 10mM dNTP, 4 microliters of 25mM MgCl2, 1 microliter of 0.1M DTT, and 1 microliter of RNaseOUT , SuperScript III reverse transcriptase 1 microliter, high-purity deionized water 4 microliters. The reverse transcription reaction was performed using an ABI PCR instrument, and the specific operating parameters were as follows: 10 minutes at 25oC; 120 minutes at 37oC; 5 minutes at 85oC; and cool to 4oC. Store at -20oC for later use. 50oC for 50 minutes, 85oC for 5 minutes, add RNaseH after cooling to 4oC, and incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com