Method for producing aluminum chloride by using fly ash
A technology of aluminum trichloride and fly ash, applied in the direction of aluminum chloride, aluminum halide, etc., can solve the problem of high cost, achieve the effects of reducing environmental pressure, improving comprehensive economic benefits, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
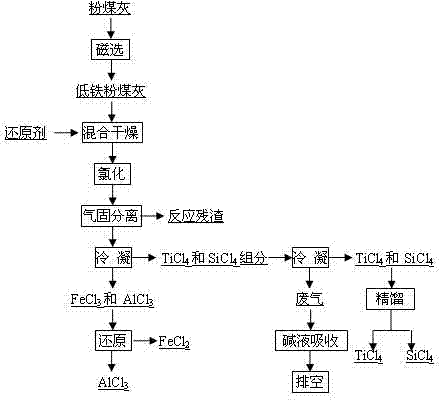
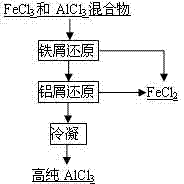
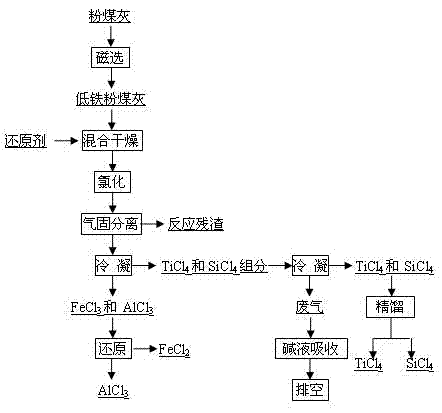
[0024] Fly ash (Al 2 o 3 53%, SiO 2 35%, Fe 2 o 3 2.0%, TiO 2 1.9%) mixed evenly with graphite powder, pressed balls, dried, chlorinated with chlorine gas for 30min at 1300°C in a fixed bed chlorination furnace, Al 2 o 3 Chlorination rate 95.6%, SiO 2 Chlorination rate 88.6%, Fe 2 o 3 Chlorination rate 97.7%, TiO 2 The chlorination rate is 95.3%, and the process chlorine utilization rate is 80%. The reaction product is cooled and separated into FeCl 3 , AlCl 3 , TiCl 4 、SiCl 4 Two groups, FeCl 3 , AlCl 3 After the mixture is heated to 480°C, it is added to a reduction furnace with iron chips for primary reduction. The temperature of the furnace is 450°C. The gas from the first reduction furnace enters the second reduction furnace with aluminum chips. The temperature of the furnace is At 400°C, AlCl with a purity of 99.6% was obtained 3 . TiCl 4 、SiCl 4 The mixture of SiCl with a purity greater than 99.90% is obtained after rectification 4 .
Embodiment 2
[0026] With fly ash (Al 2 o 3 38%, SiO 2 46%, Fe 2 o 3 6.5%, TiO 2 2.1%) as raw material, first remove iron by dry magnetic separation, 68% iron is removed during the iron removal process, Al 2 o 3 The loss rate is 5.3%. After iron removal, the fly ash and charcoal are mixed and dried, and the mixed material is chlorinated in a molten salt chlorination furnace at 750°C, and the process Al 2 o 3 Chlorination rate 92.5%, SiO 2 Chlorination rate 85.3%, Fe 2 o 3 Chlorination rate 99.8%, TiO 2 The chlorination rate is 95.2%, and the utilization rate of chlorine gas is 95%. The reaction product is cooled and separated into FeCl 3 , AlCl 3 , TiCl 4 、SiCl 4 Two groups, FeCl 3 , AlCl 3 After the mixture is heated to 400°C, it is added to a reduction furnace with iron chips for primary reduction. The temperature of the furnace is 550°C. The gas from the first reduction furnace enters the second reduction furnace with aluminum chips. The temperature of the furnace ...
Embodiment 3
[0028] Using fly ash as raw material (Al 2 o 3 49%, SiO 2 38%, Fe 2 o 3 2.1%, TiO 2 1.7%), first dry magnetic separation iron removal, 65% iron was removed during the iron removal process, Al 2 o 3 The loss rate is 5.0%. The fly ash after iron removal is added to the fluidized bed furnace in CO and Cl 2 Chlorination at 500°C in the atmosphere, process Al 2 o 3 Chlorination rate 78.1%, SiO 2 Chlorination rate 60.9%, Fe 2 o 3 Chlorination rate 88.6%, TiO 2 The chlorination rate is 81.5%, and the utilization rate of chlorine gas is 91%. The reaction product is cooled and separated into FeCl 3 , AlCl 3 , TiCl 4 、SiCl 4 Two groups, FeCl 3 , AlCl 3After the mixture is heated to 540°C, it is sent to a reduction furnace with iron chips for primary reduction. The temperature of the furnace is 600°C. The gas from the first reduction furnace enters the second reduction furnace with aluminum chips. The temperature of the furnace is 600°C. at 450°C to obtain AlCl w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com